

Strayer University. I. Hatlod, MD: "Order Cialis Extra Dosage online - Best Cialis Extra Dosage online".
About 80% of the drug was recovered unchanged in the urine after intravenous dosing purchase cialis extra dosage amex erectile dysfunction treatment lloyds, and about 60% of the drug was found in faeces after oral dosing cheap cialis extra dosage 60 mg overnight delivery impotence education. High concentrations of the drug were found in mouse kidney buy generic cialis extra dosage 50mg on-line impotence while trying to conceive, pancreas and liver, and there was low penetration to the central nervous system (Kelley et al. In three rhesus monkeys given 100 mg/kg bw zalcitabine, recovery in the urine was about 75% by five days, as in humans, but only about 9% of the drug was excreted as dideoxyuridine, which is in contrast to the human metabolic pattern. Deamination of zalcitabine to dideoxyuridine does not appear to be a significant reaction in either mice or humans but is measurable in monkeys. The studies suggest that non-human primates are an appro- priate model for studying the pharmacokinetics of zalcitabine in humans. In some of the first clinical trials, peripheral neuropathy manifested as pain, numbness and weakness occurred in 70% of patients receiving doses of ≥ 4. At the lower doses used currently, the onset of neuropathy is more gradual and the symptoms resolve more rapidly (Skowron, 1996). Of these patients, 6% had neuropathy, 14% had anaemia or neutro- penia, 6% had evidence of hepatic toxicity, 6% had stomatitis or rash and 3% had pancreatitis. Further reduction of the dose lessened the severity of symptoms but did not resolve the neuropathy. The risk factors for peripheral neuropathy were low serum cobalamin and high alcohol con- sumption (Fichtenbaum et al. A liver biopsy specimen contained macrovesicular steatosis and lobular inflammation. These are known, but rare side-effects of zidovudine that may have been exacerbated by the presence of another nucleoside analogue drug, zalcitabine (Henry et al. It has been successfully modelled in mice, rats, dogs, rabbits and monkeys (Tsai et al. Various classes of bone-marrow cells from mice given seven daily doses of 10 mg/kg bw zalcitabine were examined for 15 days after the initial exposure. The effect was greatest in committed progenitor cells of both erythroid and granulocyte–macrophage lineages and was reversible upon discontinuation of the drug (Mencoboni et al. Zalcitabine-induced regene- rative macrocytic anaemia, but no immunosuppressive effects, were found when the drug was administered to mice for up to 94 days at a dose of 2000 mg/kg bw per day (Luster et al. Rabbits treated daily for 13–18 weeks by intubation with 10–250 mg/kg bw zalci- tabine per day showed persistent lymphopenia with decreased red and white blood cell counts, haematocrit and haemoglobin concentration. Most animals had non-regene- rative macrocytic anaemia of bone-marrow origin and a progressive loss of lym- phocytes until death (Riley et al. Pigtailed macaques were given zalcitabine at 15 or 30 mg/kg bw per day intra- venously, either as a 24-h continuous infusion or a daily bolus dose for 10–12 days. All animals showed leukopenia, anaemia, lethargy and reduced appetite, and those given the bolus doses also had exfoliative dermatitis and peripheral neuropathy (Tsai et al. New Zealand white rabbits were given zalcitabine at 0–250 mg/kg bw per day for 13–18 weeks. Rabbits at doses > 50 mg/kg bw per day developed hind-limb paresis and gait abnormalities and a 30–50% decrease in nerve conduction. Electron microscopy showed demyelination of the sciatic nerve and ventral root, excess Schwann-cell basal lamina, abnormally shaped axons and the presence of lipid droplets (Feldman et al. The abnormal mitochondria were cup-shaped with tubular cristae (Feldman & Anderson, 1994). Maternal weight gain during the treatment period and gravid uterine weight were decreased at 2000 mg/kg bw per day, but weight gain corrected for gravid uterine weight was not affected. At this dose, the mean litter size was decreased, and the percentage of resorptions per litter was increased. The average fetal body weight per litter was decreased at 1000 and 2000 mg/kg bw per day. The number of fetuses with any malformation, the number of litters with one or more malformed fetuses and the percentage of malformed fetuses per litter were increased at the two higher doses. The malformations included open eyelids, micrognathia, kinked tail, clubbed paws, cleft palate, fused cervical arch, bent humerus and bent tibia (Lindström et al. References such as The Physician’s Desk Reference (Medical Economics Data Production, 1999) provide results but few or no details of the experimental conditions used in the assays. The manufacturer of the drug has yet to publish a detailed report equivalent to those available in the literature on aciclovir and zidovudine. Nevertheless, the limited geno- toxicity data available indicate that zalcitabine is clastogenic at high doses. Zalcitabine did not induce reverse mutation in Salmonella typhimurium [no information on doses or strains or the presence of exogenous metabolic activation] and did not induce gene mutation in unspecified tests in Chinese hamster lung and mouse lymphoma cells. It induced cell transformation in vitro [cell type and experimental conditions not given] at doses ≥ 500 μg/mL (Medical Economics Data Production, 1999). Zalcitabine induced prophage lambda, but not sulA, in the liquid micro- suspension assay at 1000 μg/mL. Zalcitabine caused clastogenic effects in all studies performed in vitro and in vivo, except one. Human peripheral blood cells exposed to zalcitabine with and without exo- genous metabolic activation showed increased frequencies of chromosomal aberrations at doses ≥ 1. Administration of oral doses of ≥ 500 mg/kg bw zalcitabine on three consecutive days to groups of five to seven male B6C3F1 mice produced micro- nuclei. Zalcitabine was less potent than zidovudine in inducing micronuclei (Phillips Table 1. The highest dose used in the latter study was selected to represent the daily dose of a person of average body weight, whereas patient therapy with zalcitabine, in combination with other antiretroviral agents, involves long-term treatment. The question raised by this finding is whether this low dose of zalcitabine failed to induce micronuclei in the mice or whether the genotoxic effects at these exposure levels are too small to be detected in the tests as performed (Shelby, 1994) (see section 4. Nevertheless, the occurrence of deletions in the tumours from zalcitabine-treated mice is consistent with the action of this drug as a chain terminator. In humans, the dose-limiting toxic effect, per- pheral neuropathy, requires that the daily dose be limited to about 0. The main mechanism of the antiviral activity and toxicity of zalcitabine and other ‘dideoxy-type’ nucleoside analogue drugs (see the monographs on zidovudine and didanosine, sections 4. These compounds can competitively inhibit binding of normal nucleotides to the nucleotide binding site of the reverse transcriptase and terminate replication once incorporation has occurred (Yarchoan et al. The mito- chondrial myopathy observed clinically after zidovudine therapy is not seen in patients receiving zalcitabine, perhaps because the doses are limited by the prevalence of peri- pheral neuropathy. An association between zalcitabine and peripheral neuropathy was established in a rabbit model by Feldman and Anderson (1994), who observed that rabbits with zalci- tabine-induced myelinopathy (section 4. The appearance of cup-shaped mitochondria with abnormal cristae coincided with the onset of physical symptoms. Nucleoside phosphorylation and intracellular levels of phosphorylated metabolites play an important role in zalcitabine-related toxicity. The doses at which zalcitabine induces thymic lymphomas in mice are about 1000-fold higher than the maximum doses tolerated by humans, non-human primates and rabbits. Studies of the mutagenicity of zalcitabine are scarce; however, the available data indicate that it induces clastogenic effects in vitro and in vivo at high doses.
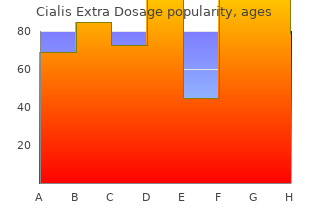
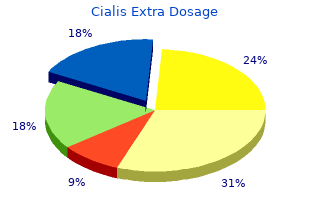
Diseases
Editorial comments • Ketamine should be used only under the strict guidance and supervision of physicians who are experienced in the adminis- tration of general anesthetics buy cheap cialis extra dosage 100mg on-line erectile dysfunction quotes. Such physicians must be knowledgeable in maintaining an airway and controlling respi- ration order cialis extra dosage 200mg on-line impotence support group. Mechanism of action: Inhibits synthesis of steroids in fungal cell membranes purchase cheapest cialis extra dosage erectile dysfunction drugs in canada, resulting in leakage of essential cellular compo- nents. Susceptible organisms in vitro: Candida sp, Cryptococcus, Coccidioides, Histoplasma, Blastomyces. Contraindications: Hypersensitivity to ketoconazole or other azole antifungals, concomitant astemizole, triazolam. Warnings/precautions • Treatment of candidiasis requires 1–2 weeks; for other sys- temic mycoses, 6 months. Clinically important drug interactions • Ketoconazole increases effects/toxicity of hepatotoxic drugs, cisapride, oral anticoagulants, astemizole, cyclosporine, astem- izole, corticosteroids, midazolam, triazolam. Mechanism of action: Inhibits cyclooxygenase, resulting in inhi- bition of synthesis of prostaglandins and other inflammatory mediators. Mechanism of Action: Inhibits cyclooxygenase, resulting in inhibition of synthesis of prostaglandins and other inflammatory mediators. Ophthalmic solution: hypersensi- tivity to any ingredient in the formulations of patients wearing soft contact lenses. Clinically important drug interactions • Drugs that increase effects/toxicity of ketorolac: alcohol, cor- ticosteroids, insulin, cimetidine, probenicid. Editorial comments: Ketorolac is recommended only for short- term use (up to 5 days) for management of moderate to severe pain. Mechanism of action: Competitive blocker of β-adrenergic receptors in heart and blood vessels. Editorial comments • Labetolol injection is intended for use only in hospitalized patients. Adjustment of dosage • Kidney disease: Creatinine clearance 30–59 mL/min: mainte- nance 150 mg/d; creatinine clearance 5–14 mL/min: initial 150 mg, maintenance 50 mg/d. Warnings/precautions: Use with caution if there is prior history or risk of developing pancreatitis, particularly in children. Stop drug administration if there are clinical or laboratory abnormal- ities suggestive of pancreatitis, kidney disease, elderly. Adverse reactions • Common: headache, insomnia, fatigue, nausea, diarrhea, vom- iting, cough, fever, chills, musculoskeletal pain. Clinically important drug interactions • The following drugs increase effects/toxicity of lamivudine: trimethoprim–sulfamethoxazole. Warnings/precautions • Use with caution in patients with the following conditions: kidney, liver, cardiac diseases. Advice to patient • Avoid driving and other activities requiring mental alertness or that are potentially dangerous until response to drug is known. Clinically important drug interactions • Drug that increases effects/toxicity of lamotrigine: valproic acid. Editorial comments: Dosage of lamotrigine is complicated by the enhancement of its metabolism by several antiepileptic drugs (phenytoin, phenobarbital, primidone) and its half-life prolongation by valproic acid. Awareness of these interactions is vital to the proper administration of this drug. Mechanism of action: Inhibits dihydroorotate dehydrogenase, resulting in inhibition of pyrimidine synthesis; also has antipro- liferative effect on T cells as well as antinflammatory actions. Contraindications: Pregnancy, significant liver disease, serology positive for hepatitis B or C. Patients with bone marrow sup- pression, severe immunodeficiency, or severe infection. Advice to patient • Use two forms of birth control including hormonal and barrier methods. Adverse reactions • Common: hypertension, diarrhea, nausea, headache, rash, res- piratory infection. Clinically important drug interactions • Drugs that increase effects/toxicity of leflunomide: folic acid, rifampin. The following method has been suggested to promote elimination of leflunomide in the event of severe toxicity: (1) discontinue leflunomide; (2) take 8 g of cholestyramine t. Mechanism of action: Drug acts as a cofactor in the biosynthesis of purines and pyrimidines which is blocked by folic acid antag- onists such as methotrexate, trimethoprim, and pyrimethamine. Leucovorin is an active metabolite of folic acid and should therefore be com- patible. Contraindications: Pernicious anemia, megaloblastic anemia secondary to vitamin B12 deficiency. Clinically important drug interactions • Leucovorin decreases effects/toxicity of trimethoprim–sulfa- methoxazole, barbiturates, phenytoin, primidone. Treatment should con- tinue until methotrexate plasma level is less than 5 × 10–8 M. Editorial comments • Parenteral leucovorin is advised for treatment of megaloblas- tic anemia due to folic acid deficiency when it is not feasible to use oral therapy. Mechanism of action: Inhibits synthesis of estrogen and andro- gens in the ovaries and testicles, thus reducing serum and tissue levels of these hormones. Should not be given to women of childbearing potential who might become pregnant during leuprolide therapy. Advice to patient • Do not stop medication without first consulting with physician. Clinically important drug interactions: Leuprolide increases effects/toxicity of antineoplastic drugs: megestrol, flutamide. Editorial comments • Leuprolide is used as palliative treatment of advanced prostate cancer when alternatives such as orchiectomy and estrogen administration are unacceptable to the patient. Mechanism of action: Relaxes bronchial smooth muscles of the bronchioles by stimulating β2-adrenergic receptors. Properties are similar to those of albuterol, and levalbuterol offers no specific advantages according to The Medical Letter. Mechanism of action: Restores depressed immune function: stimulates antibody formation, activates T cells and macro- phages. Dosage of fluorouracil is 450 mg/m2/d for 5 days, then 450 mg/m2 weekly after with 4-week break, together with 3-day course of lev- amisole starting 21–34 days following surgery. Advice to patients • Inform treating physician if you experience flu-like symptoms. Adverse reactions • Common: nausea, vomiting, diarrhea, constipation, dermatitis, alopecia, fatigue, fever, arthralgia, myalgia.
Teasel (Boneset). Cialis Extra Dosage.
Source: http://www.rxlist.com/script/main/art.asp?articlekey=96589
By the late 1970s there were a number of such companies in operation buy generic cialis extra dosage 100mg on line erectile dysfunction medications generic, including Alza buy cialis extra dosage 50 mg on-line impotence bike riding, Elan cheap 40mg cialis extra dosage with amex erectile dysfunction questions and answers, Eurand and Pharmatec International. Typically, there would be a development fee for such work, paid in stages as the project reached successive goals; finally, the client would either pay the developer royalties on the sales of the successful formulation, or subcontract production of the finished product to the advanced drug delivery company. These types of arrangements are still the basis for most development work in the advanced drug delivery sector carried out on behalf of pharmaceutical clients by specialist companies. Some of the early entrants into this field have expanded their activities into delivery routes other than their original core technology, so that they can offer solutions in the transdermal, inhalation and other fields as well as oral formulations. This is true of Alza, Elan and 3M, the latter being something of a hybrid since it is also a pharmaceutical company in the conventional sense. By contrast, some companies in this field are linked to specific routes of administration; Inhale Therapeutic systems, as its name implies, focuses on inhalation technology, while Pharmatec International, one of the oldest-established advanced drug delivery concerns, remains committed to the oral route. Drug delivery technology demands continual innovation in order to meet increasingly complex clinical demands and accommodate the needs of sophisticated new drugs. This places a heavy burden on existing specialist companies in terms of R&D commitment; it has led to the birth of a considerable number of small, research-driven concerns, often built around pharmaceutical specialists and teams from academia or the formulation departments of major pharmaceutical companies. Like companies in the biotechnology sector, these new ventures are set up to develop and exploit specific technologies, but their path to financial self- sufficiency is often shorter than that of a typical new biotech venture, because the regulatory hurdles are fewer when a new chemical entity is not involved. Here, the underlying technology is so new that it cannot even be described as “pharmaceutical” in any conventional sense. Likewise, the delivery technology is pushing back the boundaries of human knowledge, exploring the use of viruses as carriers for the genetic material, as well as other vehicles including liposomes. Since this applied research is, unusually, going hand-in-hand with fundamental research into the nature of the biological mechanisms involved, the development timetable is an extended one. In summary, the current structure of the advanced drug delivery industry is a complex one, embracing specialist companies which offer off-the-shelf and custom-developed delivery systems, some involved in a range of delivery routes, others concentrating on a single route of administration. There are leading-edge research teams in areas such as gene therapy, while some pharmaceutical concerns still maintain their own specialist advanced drug delivery formulation units developing essentially pharmaceutical solutions to formulation problems. What major contribution have advanced drug delivery systems made to anti-inflammatory drug therapy? Discuss the importance of the developing world as a market for advanced drug delivery systems. Systems are diversely referred to as “controlled release”, “sustained release”, “zero-order”, “reservoir”, “monolithic”, “membrane-controlled”, “smart”, “stealth” etc. Unfortunately, these terms are not always used consistently and, in some cases, may even be used inaccurately. For clarity and consistency, some common terms used in this book are defined as follows: • Prolonged/sustained release: the delivery system prolongs therapeutic blood or tissue levels of the drug for an extended period of time. Conventional drug delivery systems are simple oral, topical or injection formulations. Also, rate-control and drug targeting are treated as two separate issues in this book and are dealt with in detail in Chapters 4 and 5 respectively. Although there are literally hundreds of commercial products based on controlling drug release rate from delivery systems, there are in fact only a small number of mechanisms by which drug release rate is controlled: • Diffusion-controlled release mechanisms • Dissolution-controlled release mechanisms 57 Figure 3. If the drug concentration gradient remains constant, for example where solid drug particles are present and constant dissolution maintains the concentration of the drug in solution, the rate of drug release does not vary with time and zero- order controlled release is attained (see Chapter 4 and Figure 4. Diffusion-controlled reservoir devices are used in a wide variety of routes including those shown in Table 3. Regardless of a drug’s physical state in the polymeric matrix, such devices do not usually provide zero-order drug release properties. This is because as the drug molecules at the surface of the device are released, those in the centre of the device have to migrate longer distances to be released, which takes a longer time. This increased diffusion time results in a decrease in the release rate from the device with time. Generally the rate of release is found to decrease in proportion to the square root of time (“M t1/2” kinetics; see Chapter 4 and Figure 4. However, the decrease in drug release rate can be compensated for by designing systems of special geometry, which provide an increasing surface area over time. Examples of diffusion-controlled matrix devices in drug delivery are shown in Table 3. After a certain period of time the polymeric membrane dissolves, thereby releasing the drug; • matrix devices: in which the drug is distributed throughout a polymeric matrix, which dissolves with time, thereby releasing the drug. Since the dissolution of polymeric materials is the key to this mechanism, the polymers used must be water- soluble and/or degradable in water. The choice of a particular polymer for a particular controlled release dosage form depends on various factors such as the dissolution mechanism, delivery period, delivery route, the drug etc. In general, synthetic water-soluble polymers tend to be widely used for oral-controlled release dosage forms. Biodegradable polymers tend to be used for injectable, or implantable, drug delivery systems. Once the coating polymer dissolves, the drug is available for dissolution and absorption. Drug cores can be coated with polymers of different coating thickness, so that drug release can be delayed for certain periods, for example 1, 3, 6 and 12 h after administration. By using a dosage form incorporating a spectrum of different coating thicknesses, the overall drug release from the dosage form (as a whole, rather than from the individual microparticles) can adjust to give zero-order drug release. Since the size of the matrix decreases as the dissolution process continues, the amount of drug released also decreases with time. The decrease in drug release can be compensated in part by constructing a non-linear concentration profile in the polymer matrix. This strategy is used in the oral dosage form, Adalat, where the core of the dissolution matrix contains more drug than the outer layer. Microparticulates made of proteins, in particular albumin, are also widely used in the preparation of injectable drug carriers. The movement of water results in an increase in pressure in the solution and the excess pressure is known as the osmotic pressure. Osmotic pressure can used to pump out a drug at a constant rate from the delivery system. Device and formulation parameters can be controlled so that drug release is zero- order. An important consideration is that osmotic-controlled devices require only osmotic pressure to be effective, thus such devices operate essentially independently of the environment. Hence, in vitro drug release rate is often consistent with the in vivo release profile. Also, for oral delivery, changes in pH or ionic strength in the gastrointestinal tract will not affect the drug release rate. In parenteral therapy, the subcutaneously implantable, osmotic mini-pumps developed by the Alza Corp. Osmotic mini-pumps, such as the Oros osmotic pump, are also available for controlled 60 release via the oral route (see Section 6. They allow physicians and patients to precisely control the infusion rate of a drug.