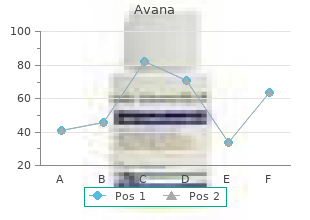


Shepherd College. I. Roy, MD: "Purchase cheap Avana online no RX - Trusted online Avana no RX".
Physicians and other caregivers could then enter orders electronically and get test results and other clinical data on their patients purchase cheapest avana erectile dysfunction treatment injection cost. However buy generic avana 50 mg on-line erectile dysfunction age 70, data storage and database management technologies were so primitive buy discount avana online erectile dysfunction cream, and computing power so modest, that it was extremely cumbersome for physicians to retrieve information at the point of care (e. Until the advent of the Apple Macintosh and, later, the Windows operating system in the mid-1980s, physicians who wanted to undertake computerized physician order entry had to learn an awkward language of computer commands and type those commands into the computer to manage their patients or to retrieve or use clinical information. As will be seen in Chapter 3, these efforts were also hampered by the highly fragmented record structure of hospitals. Hospitals 32 Digital Medicine have historically maintained separate record systems in each clinical department (for the laboratory, the operating room, the radiology department, the emergency room, etc. These so-called “legacy” systems were constructed primarily for billing purposes, not for care management. Legacy clinical systems are like a gigantic tangle of weedy undergrowth that strangles the care process as well as the efforts of those nurses, physicians, and other caregivers who use them. Even small hospitals may have as many as two dozen legacy clin- ical information systems. Unbelievably, large health systems with multiple hospitals may have as many as 500 legacy systems, pur- chased from different vendors, written in different software lan- guages, and operating on different, often incompatible hardware. As a consequence of this tangle, slightly different versions of our clinical reality exist in as many as 15 different places inside the hospital. The fact that there is no unified picture of an individual’s health status is a hazard to that person’s health. Creating a unified repository of all information requires a common format for clinical information, a single patient identifier applied across departments, and an agreement by all those who provide care to contribute what they know to the digital record. Clinical Decision Support Clinical decision support played an increasingly prominent role in emerging clinical systems. In the mid-1980s, intensive care special- ists at George Washington University led by Dr. Altogether, these tools may be the most complex commercial software products ever built, considering that they are automating what may be the most complex process in the economy—health service. Clinical systems are becoming “context aware,” meaning that they will be wired to diagnostic devices and patient monitoring equipment. They can track real-time changes in the patient’s health and will follow patients as they move through different levels of care—from an ambulatory diagnosis through surgery, into recov- ery, or even into home healthcare. These new systems now alert care providers when the patient’s condition changes, prompting the clinical team to take specific actions to deal with an emerging problem. Most importantly, however, clinical systems are reaching a suf- ficient level of intelligence to bring up-to-date medical knowledge to the physician’s office, exam room, or hospital bed. As medical science better defines how to treat patients, that knowledge will flow through computer systems to the point of care. The clinical system will prompt physicians, nurses, and others involved in patient care to follow the care pathway that holds the most promise for improving the patient’s health. The Clinician’s Role These systems do not relieve physicians and the care team of their professional and moral obligation in making patient care decisions. Just as those who use a navigational system in an airplane have Digital Medicine 35 the ultimate responsibility for reaching the destination safely, the clinical team is going to remain accountable—to patients, family members, colleagues, the courts, and society—for making the right decisions. However, clinical decision support is transforming the electronic medical record into a powerful advocate for patient safety, as well as a research tool for recording and investigating what works in medicine (Figure 2. Physicians who want to understand the basis for the system’s rec- ommendations will be able to look behind the recommendations to the research studies and clinical drug trial results and even review the outcomes of care for the last several hundred patients who received a particular treatment in the hospital to see what clinical strategies have worked best. The traditional medical record documents a patient’s health his- tory and any treatments provided. The clinical information systems presented here will be more like navigational systems in an airliner. It will locate the patient in the sphere of medical risk, constantly update the clinical team on his or her condition, and indicate a trajectory based on the latest scientific knowledge to help the care team negotiate the patient through an episode of care. The system will present a clinical “dashboard” to the physician each morning, in whatever form and venue he or she chooses (home or office desktop, portable laptop or tablet computer, or personal digital assistant). Clinical systems will be intelligent enough to rec- ognize their users by their past inquiries and even their different cognitive styles. This latter capability is especially helpful, because physicians do not all think about a medical problem the same way. Most physicians will bridle against a rigid, prepackaged approach to making care decisions. As clinical systems evolve, they will be able to recognize those cognitive differences and enable physicians or other caregivers to acquire and process information in a way with which they are comfortable. Clinical software will enable physicians to stratify their pa- tients, active and inactive, into risk groups and will both orga- nize and maintain communication with them to ensure not only that their inquiries are answered, but also that they are comply- ing with treatment recommendations. It will “remember” prescrip- tions and communicate with patients or family members about whether the therapy is producing the desired results. Clinical soft- ware will automatically schedule follow-up appointments and send patients information electronically on their illness and treatment options. Information systems will also link them automatically to disease management programs, managed by voice-response tools such as Eliza, to interact with patients to ensure that they are taking their medications as prescribed and managing their own health effectively. The remote patient monitoring systems discussed earlier, whether they are wearable devices like the wireless cardiac monitor, passive sensors like those used in the smart house, or implantable devices like Medtronic’s intelligent pacemakers, will connect “pa- tients” to physicians or the care team through their clinical infor- mation systems. We need a new term for people at medical risk that does not imply that they are institu- tionalized or under active care. Until very recently, medical science has been remark- ably incurious about what treatments actually improve the patient’s health. Safety, not efficacy, has been the principal focus both of research and of regulation. With the advent of what is now known as the Agency for Health Research and Quality in the Department of Health and Human Services, the federal government in 1989 began funding research into clinical outcomes. Additionally, more than 180 organizations, including medical and surgical specialty societies, academic health centers, and commercial companies, are developing scientifically based clinical guidelines. Natural Language Processing Another important constraint is the interface with the clinician. Although moving from typing to pointing and clicking helped make clinical software more accessible, the ability of clinicians to enter new information and interact with the system still depends more than it ought to on a mouse or keypad. Physicians do not like to type; they are used to dictating (and correcting, and reviewing, and correcting again). Removing typing or pointing and clicking from the process of interacting with the clinical system will require advances not in speech recognition, which is surprisingly powerful today, but in something called “natural language processing. Prying common meanings loose from the stream of words recognized by a computer system is the technical challenge that stands between today’s clinical systems that rely on typing or point-and-click interfaces and a truly interactive voice- response capability. According to Gartner, a respected technology evaluation firm, this capability may still be a decade off.
Practice population To maintain the health of the people in their area 100mg avana sale erectile dysfunction medication covered by insurance, physicians assess the needs of their practice population and community buy avana 200 mg lowest price erectile dysfunction doctors in nc, orient their practice to meet those needs and advocate for the health of the local community discount 100 mg avana with amex impotence and prostate cancer. Here again the physicians are using epidemiology and applying the principles of health promotion; community development and empowerment. Physicians also play a role in protecting populations from environmental and transmissible disease. As diagnosticians in direct contact with patients, they are in a unique position to identify and report unusual occurrences of disease. They are also well placed to assess possible disease sources and advise on how to reduce the spread of disease. In doing so, they are familiar with the basics of outbreak prevention and control as well as with local public health services. They borrow from management science to prioritise and implement change and to develop practice systems that improve the delivery of care. The Australian ‘Green book’ gives practical advice on how to improve delivery of preventive care, some of which can be adapted to improving patient management and follow-up (14). As part of the health system, physicians collaborate with other professionals to provide a comprehensive service. They know the resources in their area and they know how to advocate for their improvement. In so doing, they apply notions of health service organisation as well as leadership and communication skills. They also balance the needs of individuals against the needs of their practice population, employing concepts from health economics as well as applying the ethics of population medicine. Finally, physicians use the principles of infection control to prevent iatrogenic infections and cross infections between patients attending their practice. Issues specific to rural areas All these roles and responsibilities apply as much to general practice as to other branches of medicine - and as much to rural as to urban practice. However, the type, place and context of practice influence the depth of competence required in different aspects of public health. The physician in a rural general practice is likely to be one of the few health professionals in the area. In small regions, they may have privileged contact with influential people and organisations – and indeed, they may be seen as a resource for all types of health issues, including public health and community issues. This provides an excellent opportunity to advocate for health, practice health promotion and influence health protection practices and infrastructure. Changing hospital policy from the wards: An introduction to health policy education. Public health in the undergraduate medical curriculum – can we achieve integration? Public health education for medical students: Rising to the professional challenge. Towards unity for health: Challenges and opportunities for partnership in health development. Putting prevention into practice: Guidelines for the implementation of prevention in the general practice setting. Every effort has been made to ensure that the information in this chapter is accurate. This does not diminish the requirement to exercise clinical judgement, and neither the publisher nor the authors can accept any responsibility for its use in practice. Miller School of Medicine is part of the larger University of Miami Health System, which was founded in 1952 and is now home to the third-largest public hospital and third-largest teaching hospital in the United States. Serving more than one million patients every year, the hospital system extends its patient care and educational resources to South Florida, South America and the Caribbean. Big changes with small adjustments Israel Diaz, who has been with the University of Miami for over 16 years, manages the department of radiology, which consists of 50 radiologists at three hospitals. In 2012, the University of Miami transitioned its billing payment system and experienced unforeseen barriers. The radiology department endured constant backlog that, according to Diaz, “only got worse when a coder went on vacation or got sick. We had issues keeping up with the volume, so we started a small project with 3M CodeRyte CodeComplete to take care of a growing backlog of radiology notes. Miller School of Medicine But this was not Diaz’s frst experience with 3M Health Information Systems. Services provide a full-service, outsourced coding solution comprised And once they got going, Diaz says he saw immediate results. It relieved us from having to who perform medical and surgical hire and train coders. We received better coding, faster turnaround time and coding for technical, multi-specialty no hassles with stafng. With 3M CodeComplete, organizations When considering an outsourced coding solution, Diaz says that compliance can have, on average, a 48-hour was at the top of his “must-have” list. He also needed a service that had a turnaround time for processing their speedy turnaround rate. In addition to With the type of volume the department experiences, it couldn’t aford to put improved coder productivity, clients productivity at risk. Finally, compliance is the most important aspect of medical coding, and 3M is very good at compliance,” says Diaz. The results Call today By using 3M CodeComplete, Diaz says his radiology department sees remarkable For more information on how results for his team and organization. Their department has a very low denial rate 3M products and services can assist and are below their denial threshold. Once you have signed and submitted the form, your agreement with the University becomes legally binding. If the information is incorrect the University is entitled to reject your application, terminate your admission or cancel your registration immediately. Ashammakhi Summary tem cells have a capacity for self-renewal and capability of proliferation and differentiation to various cell lineages. The use of amniotic fluid derived cells, umbilical cord cells, fat and skin tissues and monocytes might be an adequate “ethically pure” alternative in future. Stem cells can improve healthcare by using and augmenting the body’s own regenerative potential. Hopefully, this will help to provide therapeutic treatment for conditions where current therapies are inadequate. Human body has an endogenous system of regeneration through stem cells, where stem cells are found almost in every type of tissue. Regenerative medicine comprises the use of tissue engineering and stem cell technology.
This was true both in relation to sales and number of companies and also in relation to public profile avana 50 mg amex tobacco causes erectile dysfunction. The situation changed abruptly generic 100 mg avana free shipping erectile dysfunction wellbutrin xl, however discount 50 mg avana amex erectile dysfunction doctors in cleveland, when biotech prod- ucts achieved their first commercial successes. In the 1990s pro- gress in gene technological and biotechnological research and development led to a veritable boom in the biotech sector. Within a few years thousands of new biotech companies sprang up all over the world. Fuelled by expectations of enormous future profits, the burgeoning biotechnology indus- try became, together with information technology, one of the driving forces behind the stock market boom of the final years of the 20th century. Measured on the basis of their stock market value alone, many young biotech companies with a couple of dozen em- ployees were worth more at that time than some estab- lished drug companies with annual sales running into hundred of millions of dollars. While this ‘investor exuberance’ was no doubt excessive, it was also essen- tial for most of the start-ups that benefited from it. For This life-size bronze sculpture of Genentech’s founders the development of a new is on display at the company’s research centre in South drug up to the regulatory San Francisco. The main reason for this is the high proportion of failures: only one in every 100,000 to 200,000 chemically synthesised molecules makes it all the way from the test tube to the pharmacy. Biotechnological production permits the manufacture of com- plex molecules that have a better chance of making it to the mar- ket. On the other hand, biotechnological production of drugs is more technically demanding and consequently more expensive than simple chemical synthesis. Without the money generated by this stock market success, scarcely any young biotech com- pany could have shouldered these financial risks. The first modern biotechnology company: Genentech It took courage to found a biotechnology company in 1976. Yet their conversation lasted three hours – and by the the search for financial rewards might endanger basic re- time it ended the idea of Genentech had been born. Itwas scarcelysurprising,therefore,that the respected developments followed rapidly: 1976 On 7th April Robert Swanson and Herbert Boyer found- ed Genentech. If these too are taken into account, the 17 Pfizer 481 following picture emerges: 18 Abbott Laboratories 397 19 Akzo Nobel 375 20 Kirin 355 Source: Evaluate Service companies or the services of contract manufacturers. As a result of the changed stock market conditions after 2000 some of these alliances evolved into takeovers: the market value of most biotech companies collapsed as abruptly as it had risen, and access to additional capital via the stock market was mostly impossible. The modern biotechnology sector is therefore now in the middle of its first wave of consolidation. Europe: Pharma enters This development did not, however, occur in the biotech sector exactly the same way all over the world. The United Kingdom, Germany, France and Scandinavia, in particular, have vibrant biotechnology sectors, while Serono, the European market leader, is a Swiss company. However the motors driving development in the world’s second most important biotech region are derived almost exclusively from the classical industrial sectors. As a supplier of laboratory equipment for use in biochem- ical research and medical diagnostics, this German company had possessed an abundance of expertise in developmental and manufacturing processes for the biotechnology sector since its very inception. It made the transition to modern bio- technology during the 1980s with the introduction of a number of recombinant (i. In a more recently developed form, this drug still plays an important role in the treatment of anemia and in oncology. This makes it one of the world’s top-selling genetically engineered medicines – and an important source of income for the company, which was integrated into the Roche Group in 1998. It be- gan large-scale production of recombinant enzymes as long ago as the early 1980s. In 1986 it introduced its first genetically en- Beer for Babylon 17 1997 1998 2001 For the first time a eukaryotic genome, The first human embryonic cell lines The first draft of the human genome is that of baker’s yeast, is unravelled. This product for use against hairy cell leukemia was manufactured under li- cence from Genentech. After its takeover of Boehringer Mannheim, Roche devel- oped the Penzberg site into one of Europe’s biggest bio- technology centres. Finally, its ac- quisition of a majority stake in the Japanese pharmaceu- ticalandbiotechnology com- pany Chugai in 2002 put the Roche Group close behind the world market leader Amgen in terms of biotech sales. Its competitors have fol- lowed a similar course, though in some cases later or with different focuses. Boehringer & Söhne, under- first recombinant drug to be discovered, developed and pro- takes biochemical work in the former Hotel Simson in Tutzing. The resulting expertise has paid off: The Roche Group Syntex and in 1995 converts it into Roche Biosciences. Roche’s returns 42% of the company’s shares to the stock market; the Diagnostics Division supplies over 1700 biotechnology-based monoclonal antibody Herceptin is approved for use in breast products. Key milestones on the way to this success 2000 The Basel Institute for Immunology is transformed in- are listed below: to the Roche Center for 1896 Fritz Hoffmann-La Roche founds the pharmaceutical Medical Genomics. Japan: potential in Compared to their counterparts in Europe, the biotechnology pharmaceutical companies of the various Asian countries – which are otherwise so enthusiastic about new technology – were slow to recognise the potential of this new industrial sector. This despite the fact that the Japanese pharmaceutical market is the world’s second largest, after that of 20 Number one in Japanese biotechnology: Chugai Pharma 1925 Juzo Uyeno founds a small pharmaceutical company in Tokyo that becomes increasingly impor- tant nationally over the coming decades. A few years ago the Japanese phar- in Japan and later also in Europe, Australia and China. Roche, Chugai has become not only the fifth largest pharma- 1997 Chugai Diagnostics Science is formed. Moreover, two Japanese companies, Takeda and Sankyo, rank among the 20 largest pharmaceutical companies in the world. In the 1990s Japan set out on the road to catch up, in particular via large-scale support programmes and targeted alliances. The result is that Japanese pharmaceutical companies are now at least on a par with their counterparts in most European coun- tries in terms of sales of biopharmaceutical products. However, the country still lags behind in terms of the number of biotech companies based there, the period of rapid expansion in the 1990s having largely passed Japan by. As yet,Japanese companies devoted exclusively to modern biotechnology have an even smaller slice of the world market than their European competi- tors. Japanese biotechnology is largely in the hands of representatives of classical branches of industry such as the brewery Kirin, the food manufacturer Takara, the chemical manufacturer Kyowa Hakko and variouspharmaceutical companies. Themarket lead- er in modern biotechnology in Japan is Chugai Pharmaceutical Beer for Babylon 21 Co. Milestones along this company’s development in this area were its acquisition of the American biotech company Gen-Probe in 1989 and, a year later, the granting of regulatory approval for its first genetically engineered drug, Epogin (active ingredient: erythropoietin, for use in anemia). Access to the worldwide market for these products is provided by the Roche Group, which acquired a majority stake in Chugai in 2002. The merger between Nippon Roche, Roche’s Japanese subsidi- ary, and Chugai in 2002 led to the formation of Japan’s fifth- largest pharmaceutical company and largest biotech company.