

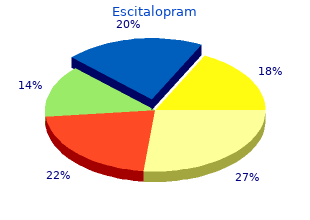
William Carey University. W. Derek, MD: "Buy online Escitalopram - Safe online Escitalopram".
For adverse events we included both experimental and observational studies discount escitalopram online amex acute anxiety 5 letters. For observational studies cheap escitalopram line anxiety 8 year old boy, we included those with large sample sizes (≥ 100 patients) order 5mg escitalopram amex anxiety 9 year old boy, lasting at least 1 year that reported an included outcome. Initially, we reviewed studies with health outcomes as primary outcome measures. Outcomes for efficacy or effectiveness were response, remission, speed of response, relapse, functional capacity, and hospitalization. If no study measuring health outcomes was available for a particular indication or population subgroup, we included intermediate outcomes (e. Safety outcomes included overall and specific adverse events (e. We included meta-analyses in our evidence report if we found them to be relevant for a 10 key question and of good or fair methodological quality. We did not review individual studies if they were included in a high-quality meta-analysis. We excluded meta-analyses that were not based on a comprehensive systematic literature search or did not maintain the units of the studies in their statistical analyses. We checked our database to guarantee that our literature search had detected trials included in any meta-analyses that we discarded, and we then obtained any missing articles. Data Abstraction We designed and used a structured data abstraction form to ensure consistency of appraisal for each study. Trained reviewers abstracted data from each study and assigned an initial quality rating. A senior reviewer read each abstracted article, evaluated the completeness of the data abstraction, and confirmed the quality rating. We abstracted the following data from included trials: study design, eligibility criteria, intervention (drugs, dose, duration), additional medications allowed, methods of outcome assessment, population characteristics, sample size, loss to follow-up, withdrawals due to adverse events, results, and adverse events reported. We recorded intention-to-treat results if available. Quality Assessment We assessed the internal validity (quality) of trials based on predefined criteria (Appendix B). These criteria are based on those developed by the US Preventive Services Task Force (ratings: 11 12 good-fair-poor) and the National Health Service Centre for Reviews and Dissemination. External validity (generalizability) was assessed and reported but did not influence quality ratings. Two independent reviewers assigned quality ratings; they resolved any disagreements by discussion and consensus or by consulting a third, independent party. Elements of internal validity assessment included, among others, randomization and allocation concealment, similarity of compared groups at baseline, use of intention-to-treat analysis, and overall and differential loss to follow-up. Loss to follow-up was defined as the number of persons randomized who did not reach 13 the endpoint of the study, independent of the reason and the use of intention-to-treat analysis. We adopted a cut-off point of 20 percent loss to follow-up as a limit beyond which bias was Second-generation antidepressants 14 of 190 Final Update 5 Report Drug Effectiveness Review Project likely to be introduced because of missing endpoint assessments. Trials with more than 20 percent but less than 40 percent loss to follow-up were eligible for a quality rating of fair (but not good). Studies with more than 40 percent overall loss to follow-up or more than 15 percentage points differential loss to follow-up between study groups were rated as poor. These cut-off points took into consideration that loss to follow-up appears to be higher in psychiatric populations than in other study populations. Trials that had a fatal flaw in one or more categories were rated poor quality and not included in the analysis of the evidence report (Appendix C) unless the evidence was severely lacking for an indication. Trials that met all criteria were rated good quality. The majority of trials received a quality rating of fair. This includes studies that presumably fulfilled all quality criteria but did not report their methodologies to an extent that answered all our questions. Thus, the “fair quality” category includes trials with quite different strengths and weaknesses. The results of some fair quality studies are likely to be valid; others are probably valid. Data Synthesis We conducted meta-analyses of data for head-to-head comparisons for trials that were fairly homogenous in study populations and outcome assessments. Our outcome measure of choice was the relative risk (RR) of being a responder on the Hamilton Rating Scale for Depression (HAM-D) or the Montgomery-Asberg Depression Rating Scale (MADRS) (more than 50 percent improvement from baseline) at study endpoint. We chose this outcome measure because response to treatment can be viewed as a close proxy to health outcomes. Therefore, such an outcome measure has more clinical significance than a comparison of mean changes of scores on rating scales. For each meta-analysis, we conducted a test of heterogeneity and applied both a random and a fixed effects model. We report the random effects model results because, in all three meta- analyses, the results from random and fixed effects models were very similar. If the RR was statistically significant, we then conducted a meta-analysis of the risk differences to calculate the number needed to treat (NNT) on the pooled risk difference. We assessed publication bias using funnel plots and Kendell’s tests. However, given the small number of component studies in our meta-analyses results of these tests must be viewed cautiously. All statistical analyses were conducted using StatsDirect, version 2. Second-generation antidepressants 15 of 190 Final Update 5 Report Drug Effectiveness Review Project RESULTS Overview We identified 4,850 (1637) citations from searches and reviews of reference lists. We identified an additional 40 citations from dossiers submitted by pharmaceutical companies and 6 from public comments. Some citations were reported in abstract form only and were subsequently excluded (Appendix D). In all, we included 275 (59) studies: 170 (13) RCTs, 40 (13) meta-analyses, 39 (15) observational studies, and 14 (4) studies of other design. Furthermore, we retrieved 175 (83) articles for background information. Five (Three) studies of interest could not be retrieved after 14-18 multiple attempts. Figure 1 (PRISMA flow chart) documents the disposition of the 1067 (278) articles for these studies. Reasons for exclusions were based on eligibility criteria or methodological criteria (Figure 1, PRISMA flow chart). Seventy-two studies (75 articles) that met the eligibility criteria were later rated as poor quality for internal validity and excluded from the analysis (Appendix C). The two main reasons for a poor quality rating among RCTs were high loss to follow-up (more than 40%) and lack of double-blinding. Among meta-analyses, lack of a systematic literature search was the main reason for exclusions.
Theseobservations on mice and humans support the hypothesis that individuals have narrowly focused antibody memory and that individu- als vary in the antigenic sites to which they respond cheap escitalopram 20mg fast delivery anxiety symptoms gi. This combination of individual focus and population variability creates a heterogeneous pat- tern of selection onparasites order escitalopram overnight anxiety symptoms questionnaire. After a widespread epidemic by a single parasite type discount escitalopram 5mg on-line 8 tracks anxiety, the parasite must acquire several new mutations before it can again spread widely through the population. Stepwise changes can occur by first changing at one site and attacking a subset of the population with a dominant response against that site. The new mu- tant strain can then accumulate a second change that provides access IMMUNOLOGICAL VARIABILITY OF HOSTS 135 both to hosts with a dominant antibody response to the second mutant site and to hosts with antibodies against both the first and second mu- tant sites. Additional mutations allow attack against broader sets of immunological profiles. This description certainly oversimplifies the actual process. However, the immunodominance of individual hosts for particular epitopes and the population variability of immune profiles can create important se- lective pressures on parasites. Typically,memoryleads to a faster and more vigorous secondary response. Suppose, however, that a host first de- velops a memory response to a particular antigen, and then is exposed secondarily to a variant ofthatantigen. If the secondary variant cross- reacts with memory cells, then the host may produce a memory response to the first antigen rather than a primary response to the second antigen. Amemoryresponse to the first antigen rather than a primary response to the variant is called original antigenic sin. Amemoryresponse based on previously encountered, cross-reactive antigens has three consequences for the immunological structure of host populations. First, cross-reaction may aid protection or clearance against secondary challenge. This occurs if the cross-reactive memory effectors have sufficient affinity for the variant antigen (Kaverin et al. Second, cross-reaction may interfere with the secondary response. This occurs when cross-reactive memory effectors do a poor job of clear- ing secondary challenge but respond sufficiently to repress a new, pri- mary response against the variant antigen (Good et al. Third, the host may fail to develop an increasingly broad memory profile over the course of repeated exposures to different variants. This occurs when a new variant stimulates cross-reactive memory rather than aspecificprimary response, preventing memory particular for the new 136 CHAPTER 9 variant (Fazekas de St. Ihavealready mentioned the immunodominance of individual immune profiles and the tendency for the pattern of immunodominance to vary among individuals. I also discussed how cross-reactivity can affect clear- ance of secondary challenge and the development of memory over a host’s lifetime. In this section, I add a few more factors that affect the distribution of immune profiles. AGE STRUCTURE OF HOSTS An individual becomes exposed over time to an increasingly diverse array of parasite genotypes. Thus, older individuals typically have a broader memory profile than do younger individuals. Age-related pat- terns have been measured by serological surveys, which describe the presence or absence of circulating antibodies to a particular strain of parasite or to a particular antigen. Many surveys have been published forawide variety of parasites and hosts (Anderson and May 1991, pp. Here are just a few example pathogens for which broader immunolog- ical profiles have been reported in older hosts compared with younger hosts: influenza (Dowdle 1999), Plasmodium (Gupta and Day 1994; Bar- ragan et al. The best data on age effects come from studies of the influenza A virus. Most neutralizing antibodies against influenza bind to hemag- glutinin, the virus’s dominant surface molecule (Wilson and Cox 1990). Three major subtypes of hemagglutinin have circulated in human pop- ulations since about 1890, labeled H1, H2, and H3. Antibodies to one subtype cross-react relatively littlewiththeother subtypes. Although antibodies to a partic- ular variant do not always protect against infection by other variants of the same subtype, the antibodies to variants of a subtype do often cross-react to some extent. IMMUNOLOGICAL VARIABILITY OF HOSTS 137 100 (a) 90 A/Swine/15/30 (H1) 80 70 60 50 40 30 20 10 0 100 (b) 90 80 70 60 A/Hong Kong/68 (H3) 50 A/Japan/57 (H2) 40 30 20 10 0 Year of Birth Figure 9. The strains labeled A/strain des- ignation (subtype) were used to test for antibodies to a particular subtype by measuring the degree to which blood samples carried antibodies that reacted significantly against the test strain. Figures were taken from Dowdle (1999), with permission from WHO. Original data for the top panel from Masurel (1976, with permission from Elsevier Science) and for the bottom panel from Masurel (1969, with permission from WHO). These patterns of cross-reaction allow one to measure immunological profiles of individuals with regard to previous exposure to each of the three subtypes. By measuring individuals of different ages, a picture emerges of the past history of exposure and immunity to the different subtypes. H1 is the subtype that caused the famous 1918 138 CHAPTER 9 5 October–December 1957 (H2) 4 December–January 1969 (H3) 3 2 1 0 5 15 25 35 45 55 65 75 85 Age (years) Figure 9. The 1957 pandemic was caused by an H2 subtype and the 1968–69 pandemic was caused by an H3 subtype. Fig- ure taken from Dowdle (1999), with permission from WHO. Original data from Housworth and Spoon (1971), with permission from Oxford University Press. Note that antibodies against H1 occur in 80–90% of individuals who were less than twenty years old during the pandemic years, suggesting widespread dis- tribution of the disease. The drop in the seropositive level for individu- als born before 1900 may be explained by the typically lower percentage of adults than children infected by influenza epidemics (Nguyen-Van- Tam 1998). There may also be some decay in immune memory among olderindividuals. The largedropinseroprevalence after 1922 suggests that H1 declined in frequency after the pandemic. Perhaps because of widespread immunity to H1, variants of this subtype had difficulty spreading between hosts. Cohorts born in the years before the pandemic had very high seroprevalence, suggesting widespread infection.
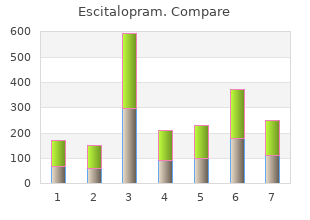
Order 5 mg escitalopram amex. Alpha-Stim M Application for Pain Anxiety Depression & Insomnia.
Syndromes