

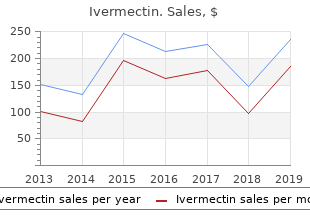
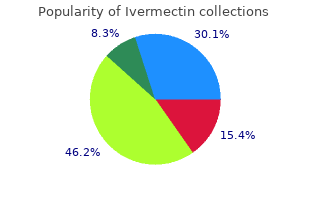
Mercer University. A. Kippler, MD: "Buy online Ivermectin cheap - Discount online Ivermectin no RX".
The following section describes the appropriate location for drug metabolism and drug-drug interaction information cheap 3mg ivermectin fast delivery antibiotics zinnat. The role of P-gp and other transporter mechanisms and their relationship to drug-metabolizing enzymes remain to be fully understood order ivermectin pills in toronto antibiotic premedication for dental procedures, and the effects on P-gp-mediated transport are only beginning to be reflected in labeling at this time order line ivermectin bacteria for kids. It is easy to envision, however, that the role of transporters and the clinical consequences of their modulation will soon be better understood and studied so that information on these systems will appear regularly in labeling. The clinical consequences of metabolism and interactions should be placed in drug interactions, warnings and precautions, boxed warnings, contraindications, or dosage and administration sections, as appropriate. Information related to clin- ical consequences should not be included in detail in more than one section, but rather reported fully in one section and then referenced in other sections, as appropriate. When the metabolic pathway or interaction data results in recom- mendations for dosage adjustments, contraindications, or warnings (e. Refer to the guidance for industry on labeling (32) for more information on presenting drug interaction information in labeling. In certain cases, information based on clinical studies not using the labeled drug can be described with an explanation that similar results may be expected for that drug. The information provided by these studies needs to be appreciated and understood by prescribers and utilized in individualizing pharmacotherapy. An integrated approach to studying and evaluating drug-drug interactions during the drug development and regulatory review process and incorporating language into labeling has been described. This integrated approach should be based on good under- standing and utilization of the primary question, our willingness to rely on in vitro and in vivo pharmacokinetic and pharmacodynamic data, and our understanding of the degree to which an observed change in substrate mea- sures caused by an interacting drug is or is not clinically important. In recent years, understanding the metabolic disposition and identifying the potential for metabolic drug-drug interactions such as inhibition and induction of enzymes has become an integral part of the drug development process. Improved understanding of the mechanistic basis of metabolic drug-drug interactions has enabled standardized and focused approaches to evaluating interactions with generalizable conclusions. The recently published guidance (10) reflects the agency’s current view in the evaluation of drug- drug interactions during drug development and includes the following prin- ciples. Future efforts in assessing, managing, and communicating the risks of drug-drug interactions may focus on (1) improved uses of in vitro tests to evaluate transporter-based interactions, (2) better use of in vitro data as a surrogate for in vivo findings, e. Lesko, Patrick Marroum, Srikanth Nallani, Janet Norden, Wei Qiu, Atik Rahman, Kellie Reynolds, Soloman Sobel, Toni Stifano, John Strong, Robert Temple, Kenneth Thummel, Douglas C. Drug interaction studies—study design, data analysis and implications for dosing and labeling. Inhibition of P-glycoprotein-mediated drug transport: a unifying mechanism to explain the interaction between digoxin and quinidine. Sildenafil citrate and blood-pressure-lowering drugs: results of drug interaction studies with an organic nitrate and a calcium antagonist. Life-threatening interaction of mibefradil and beta-blockers with dihydropyridine calcium channel blockers. Draft guidance for industry: Drug-Drug interactions— study design, data analysis and implications for dosing and labeling. Changes in the pharmacokinetics and electrocardiographic pharmacodynamics of terfenadine with concomitant admin- istrations of erythromycin. Assessment of single- and multiple-dose interactions between ritonavir and saquinavir. Drug-drug, drug-dietary supplement, and drug- citrus fruit and other food interactions—labeling implications. Effects of gemfibrozil, itraconazole, and their combination on the pharmacokinetics and pharmacodynamics of repaglinide: potentially hazardous interaction between gemfibrozil and repaglinide. The safety of newly approved medicines: do recent market removals mean there is a problem? What have we learned from the recent market withdrawal of terfenadine and mibefradil? Presentation at the 101st Annual Meeting of American Society of Clinical Pharmacology and Therapeutics, March 15–17, 2000, Beverly Hills, California. Guidance for Industry: Exposure-Response Rela- tionship, Study Design, Data Analysis, and Regulatory Applications, April 2003 (posted May 2003). Appropriate phenotyping procedures for drug metabolizing enzymes and transporters in humans and their simultaneous use in the ‘‘Cocktail’’ Approach. Nelson Department of Medicinal Chemistry, School of Pharmacy, University of Washington, Seattle, Washington, U. Most adverse drug reactions occur in only a small percentage of patients and are termed idiosyncratic, and many of these reactions are caused by reactive metabolites formed from drugs (2–4). Reactions of reactive metabolites with tissue macromolecules can lead to direct or intrinsic toxic effects and/or cause toxicity by forming haptens that lead to immunotoxic effects. Although new animal models are being developed that provide insights into factors that play a role in these idiosyncratic toxicities (5–7), no generally useful models are yet available. In some cases a new drug may be the precipitator or perpetrator of toxicity of another drug by altering its metabolism and/or disposition, or the new drug may be the object or victim of altered metabolism and/or disposition caused by a drug already on the market. In many instances, the object or victim is a drug with a narrow therapeutic index, window, or ratio (for a discussion, see Ref. Several definitions have been applied to this terminology, including the qual- itatively simple one of a drug ‘‘for which relatively small changes in systemic 687 688 Nelson concentrations lead to marked changes in pharmacodynamic response’’ (9). This chapter will focus on those metabolic drug-drug interactions that have led or can lead to serious toxicological consequences in humans. Most of the chapter will describe examples of metabolic drug-drug interactions that have caused serious toxicities. As discussed in chapter 15, the majority of drug-drug inter- actions of clinical significance have occurred through interactions at the level of cytochromes P450. Since substantial information is now either available or readily obtainable about induction and inhibition of these enzymes as well as the kinetic parameters associated with the metabolism of drugs and other probe substrates, many metabolic drug-drug interactions can be predicted prior to clinical trials. However, because the situation in vivo is complicated by a variety of genetic and environmental factors that affect drug absorption, distribution, and metabolism and because the physiological response to a toxic insult may vary from one individual to another, it is often difficult to predict that a particular drug-drug interaction will lead to a toxic insult. Nonetheless, the results of preclinical studies should provide the basis for more informed planning of clinical studies. Warfarin Because of its narrow therapeutic window and extensive oxidation to inactive metabolites by cytochromes P450, warfarin (and the closely related drug ace- nocoumarol) is subject to many metabolic drug-drug interactions that can place patients at severe risk of either hyper- or hypocoagulability. Several inducers of cytochromes P450, including rifampin, several barbi- turates, aminoglutethimide, primidone, phenytoin, and carbamazepine increase requirements for warfarin dosing, although mechanisms for most of these interactions have not been thoroughly investigated (11–13). Clinically, this effect becomes manifest either when a patient stabilized on warfarin adds one or more of these drugs to his or her therapy or, more commonly, when the patient removes one of these drugs from his or her therapy after stabilization on the combination therapy. Several inhibitors of cytochromes P450 can substantially decrease require- ments for warfarin dosage that, if not attended to, can lead to life-threatening bleeding episodes. Cimetidine contains an imidazole moiety, but it is a much better inhibitor of the metabolism of (R)-warfarin (17), the least potent enantiomer, so that an effect on warfarin therapy is observed only at high doses of cimetidine (18). Although many case reports have appeared of interactions between warfarin and a variety of other drugs with many different drug structures (19), only a few of these have resulted in serious toxic effects, and mechanisms are largely unknown. Because of their increased use, further investigations with some of these drugs, such as tamoxifen (20,21), seems warranted.
A 5-year-old child becomes ill while visiting relatives who have a farm in Arkansas purchase ivermectin master card infection 3 weeks after wisdom teeth removal. His symptoms include severe abdominal cramps with vomiting and diarrhea and profuse lacrimation and salivation effective 3mg ivermectin antibiotics before tooth extraction. If these symptoms are due to chemical toxicity order ivermectin 3 mg with visa antibiotic levo, the most likely cause is exposure to A. The activation of muscarinic receptors in bronchiolar smooth muscle is associated with A. Overuse of certain decongestants that are indirect-acting sympathomimetics can lead to a diminished response. With this principle in mind, one can anticipate that hexa- methonium will cause A. The presumptive diagnosis was drug toxicity due to the ingestion of a compound similar to A. Reflex tachycardia is most likely to occur after the systemic administration of A. Which one of the following sites is characterized by adrenergic neurohumoral transmis- sion? Activation of prejunctional 0:2 receptors on sympathetic nerve endings is associated with A. The data in the table below show the effects of four drugs (#1-4) on mean blood pressure administered as individual agents before and after treatment with prazosin. Ocular effects that include mydriasis and fixed far vision are characteristic of A. Following a myocardial infarct, a 40-year-old male patient is being treated prophylacti- cally with propranolol. In terms of adverse effects of the drug, which of the following is most likely to occur with use of this specific beta blocker? A 45-year-old Nobel Prize-winner in chemistry has recently been the recipient of a heart transplant. Patient education has included both verbal and written descriptions of the potential cardiovascular effects of pharmacologic agents. Which one of the following drugs is least likely to cause tachycardia in this patient? Because you know about the problem of distinguishing between cholinergic excess and under- treatment in the myasthenic patient, you would probably recommend that your colleague be given A. A number of pharmacologic treatments can slow the progression of the dis- ease, which can ultimately lead to complete blindness if left untreated. Beta blockers cause ciliary muscle contraction, increasing aqueous humor outflow B. Labetalol is an effective antihypertensive agent that, like propranolol, is capable of block- ing beta receptors. Antagonist Pretreatment Agonist 1 Agonist 2 Agonist 3 J, J, None r Atropine r - r Prazosin i - r J, J, Propranolol - Mecamylamine t - r Identify the agonist drugs from the following list: A. Bradycardia due to vagal stimulation is elicited by activation of muscarinic receptor in the heart. Pilocarpine is present in several mushroom species including Amanita mU5- caria, the ingestion of which is associated with the stimulation of M receptors (parasym- pathomimetic effects). All of the other effects listed are typical of excessive stimulation of M receptors. Urinary retention is a well known adverse effect of drugs that have antagonist effects on muscarinic receptors. In addition to the prototypic drug atropine, M blockers include drugs used in Parkinson disease, such as benztropine. The symptoms of cholinergic excess seen in this child are indicative of exposure to insecticides such as the organophosphate parathion, which cause irreversible inhibition of acetylcholinesterase. Muscarinic receptors present in bronchiolar smooth muscle are of the M 3 subtype coupled via Gq proteins to phospholipase C. Tachyphylaxis, a rapid loss of pharmacologic activity, frequently occurs with indirect-acting sympathomimetics such as amphetamine, ephedrine, and pseudoephedrine. The signs and symptoms experienced by this boy are highly suggestive of the ingestion of a compound with strong muscarinic receptor blocking actions. The leaves and seeds of jimsonweed (Datura stramonium) contain anticholinergic compounds, including atropine, hyoscyamine, and scopolamine-approximately 50 seeds may cause severe toxicity. Although used primarily via inhalation for asthma, systemic effects of albuterol include vasodilation due to its ~2 receptor activation. Methoxamine and phenylephrine are (X- receptor activators causing vasoconstriction, which would result in reflex bradycardia. Ganglion blockers (mecamylamine) prevent autonomic reflexes, and a reflex increase in heart rate could not occur in the presence of a beta blocker (propranolol). A decrease in mean blood pressure, an increase in pulse pressure, plus a marked increase in heart rate are characteristic of a drug such as isoproterenol. A similar mechanism involving Gi protein inhibition of adenylyl cyclaseoccurs with activation of M2 receptors (see answer 6). Of the drugs listed, only isoproterenol causes a decrease in mean blood pres- sure, because it activates beta receptors and has no effect on alpha receptors. Prazosin is an alpha blocker, so one can antici- pate that this drug would antagonize any increases in blood pressure that result from acti- vation of (Xl receptors in the vasculature. Epinephrine (high dose), norepinephrine, and tyramine all exert pressor effects via activation of (Xl receptors. However, only epinephrine is active on ~2 receptors, and this action would be revealed by vasodilation and a reversal of its pressor effects following treatment with an alpha blocker-"epinephrine reversaL" Thus, drug #4 can be identified as epinephrine. Mydriasis and fixed far vision can be due to either muscarinic receptor antagonists or ganglionic blockade. The cholinesterase inhibitor (neostigmine) and alpha blocker (phentolaminelcause miosis. Ocular effects of the beta blocker (timolol) are restricted to decreased formation of aqueous humor by the ciliary epithelium. Mydriasis is associated with blockade of M receptors, and both micturition and sweating result from activation of such receptors. However, reflex bradycardia is not possible following pretreatment with an M blocker. This question is to remind you that indirect-acting sympathomimetics require innervation of the effector organ to exert effects. However, transplanted hearts retain receptors, including those (~l) responsive to direct-acting sympathomimetics. If symptoms improve with a single dose of edrophonium, then an increase in the dose of neostigmine or pyridostigmine is indicated. The effectiveness of labetalol in the management of hypertension and in severe hypertensive states appears to be due to a combination of antagonistic actions at both alpha and beta adrenoceptors. Labetalol is not a ~l selective blocking agent (unlike atenolol and metoprolol), and (unlike pindolol and acebutolol) it lacks intrinsic sympa- thomimetic activity. Labetalol is available for both peroral and parenteral use; unfortu- nately, it blocks ~2 receptors in bronchiolar smooth muscle.
These developments have led to the birth of a new economic sec- tor order ivermectin 3mg otc virus uncoating, the biotech industry order genuine ivermectin antibiotics for a sinus infection, associated mostly with small start-up companies buy 3 mg ivermectin virus affecting children. For their part, the more established healthcare com- panies have also been employing these modern techniques, known collectively as biotechnology, successfully for many years. By studying the molecular foundations of diseases they have developed more specific ways of combating diseases than ever before. This new knowledge permits novel approaches to treatment, with new classes of drug – biopharmaceuticals – at- tacking previously unknown targets. Increasing attention is also being paid to differences between individual patients, with the result that in the case of many diseases the goal of knowing in advance whether and how a particular treatment will work in a given patient is now within reach. When a disease, rather than being diagnosed on the ba- sis of more or less vague signs and symptoms, can be detected on the basis of molecular information, the possibility of suc- cessful treatment depends largely on what diagnostic techniques are available. To the healthcare industry this represents a major development in that diagnosis and treatment are growing ever closer together, with clear benefits for companies that possess competence in both these areas. To patients, progress in medical biotechnology means one thing above all: more specific, safer and more successful treatment of their illnesses. For example,more than 40% of the sales of Roche’s ten best-sell- ing pharmaceutical products are currently accounted for by bio- pharmaceuticals, and this figure is rising. This booklet is intended to show what has already been achieved via close cooperation between basic biological research, applied science and biotechnologically based pharmaceutical and diag- nostic development. Just as in the past the development of beer, bread and cheese were major breakthroughs, another revolution is now about to overtake medicine: compounds produced using biotechnological methods are opening up entirely new possibilities in medical diagnostics and therapy, and in so doing are bringing about a major restructuring of markets. From knowledge to science: the history of biotechnology Babylonian biotechnologists were a highly regarded lot. Their products were in demand among kings and slaves and were ex- ported as far as Egypt. They are even mentioned in the Epic of Gilgamesh, the world’s oldest literary work – the Babylonian brewers, with their 20 different types of beer. Their knowledge was based on a biological technology that was already thousands of years old – fermentation Terms by yeast. Biopharmaceuticals drugs manufactured using biotech- Though it may sound nological methods. The only thing that is relatively new about the biotechnology industry is its name. Stone Age, Iron Age, The term ‘biotechnology’ was first used in a 1919 Age of Biochemistry publication by Karl Ereky, a Hungarian engineer and economist. He foresaw an age of biochemis- try that would be comparable to the Stone Age and the Iron Age in terms of its historical significance. For him, science was part of an all-embracing economic theory: in combination with po- litical measures such as land reform, the new techniques would provide adequate food for the rapidly growing world population – an approach that is just as relevant today as it was in the pe- riod after the First World War. Until well into the second half of the 20th century biologists worked in essentially the same way as their Babylo- Beer for Babylon 9 1665 C. Two years later Antoni van Leeuwenhoek becomes the first person to see bacterial cells. Thanks to newly developed methods, however, the biotechnol- ogy of the 20th century was able to produce a far greater range of such natural products and at far higher levels of purity and quality. This was due to a series of discoveries that permit- ted the increasingly rapid development of new scientific tech- niques: T In the first half of the 19th century scientists discovered the basic chemical properties of proteins and isolated the first enzymes. Over the following decades the role of these sub- stances as biological catalysts was elucidated and exploited for research and development. T The development of ever more sophisticated microscopes rendered the form and contents of cells visible and showed the importance of cells as the smallest units of life on Earth. Louis Pasteur postulated the existence of microorganisms and believed them to be responsible for most of the fermen- tation processes that had been known for thousands of years. T From 1859 Charles Darwin’s theory of evolution revolution- ised biology and set in train a social movement that led ul- timately to a new perception of mankind. For the first time the common features of and differences between the Earth’s organisms could be explained in biological terms. As a result, biology changed from a descriptive to a more experimental scientific discipline. T The rediscovery of the works of Gregor Mendel at the end of the 19th century ushered in the age of classical genetics. Cultivation and breeding techniques that had been used for thousands of years now had a scien- tific foundation and could be further developed. It will be white blood cells in purulent 35 years before his work receives the bandages that he refers to as ‘nuclein’. In addition to the classical, mostly agricultural, products, more and more new products entered the market- place. Enzymes were isolated in highly purified form and made available for a wide variety of tasks, from producing washing powder to measuring blood glucose. Standardised biochemical test methods made their entrance into medical diagnostics and for the first time provided physicians with molecular measuring instruments. The structures and actions of many biomolecules were elucidated and the biochemical foundations of life thereby made more transparent. Gene technology spurs However,it was only with the advent of gene tech- innovation nology that biology and biotechnology really took off. Desired changes in the genetic makeup of a species that previously would have required decades of system- atic breeding and selection could now be induced within a few months. For example, newly developed techniques made it possible to in- sert foreign genes into an organism. This opened up the revolu- tionary possibility of industrial-scale production of medically important biomolecules of whatever origin from bacterial cells. The first medicine to be produced in this way was the hormone insulin: in the late 1970s Genentech, an American company, de- veloped a technique for producing human insulin in bacterial cells and licensed the technique to the pharmaceutical company Eli Lilly. Gene technology: human insulin from bacteria In 1982 human insulin became the world’s first biotechnolog- In 1978 the biotech company Genentech developed a method ically manufactured medicine. These were then separately isolated, combined and betes and most people with type 2 diabetes require regular finally converted enzymatically into active insulin. In its day, this classical biotechnological method it- Some 200 million diabetics worldwide now benefit from the self represented a major medical breakthrough: until 1922, production of human insulin. Without gene technology and when medical scientists discovered the effect of pancreatic biotechnology this would be impossible: in order to meet cur- extracts, a diagnosis of type 1 diabetes was tantamount to a rent demands using pancreatic extract, around 20 billion pigs death sentence. A new economic This technology laid the foundation for a new in- sector arises dustry. The early start-up biotech companies joined forces with large, established pharmaceu- tical companies; these in turn used biotechnology to develop high-molecular-weight medicines. Rapid expansion In the early 1980s very few companies recognised and stock market boom the medical potential of the rapidly expanding field of biotechnology.
Discount ivermectin 3mg overnight delivery. Outcome of Antimicrobial Stewardship - What Metrics are Worth Measuring.
Transport Via the Large Neutral Amino Acid Transporter Is Affected by Diet The pharmacological effect of L-dopa is affected by diet (362) cheap ivermectin master card infection 7 weeks postpartum. The ‘‘off’’ period in Parkinsonian patients treated with L-dopa is a clinical problem buy online ivermectin infection joint pain, since the efficacy of the drug suddenly fails generic 3mg ivermectin antibiotic tendon rupture. In addition to L-dopa, baclofen and melphalan are suggested to be taken up into the brain via amino acid transporter (363,364), and thereby, their brain transport might be also affected by the plasma concentration of large neutral amino acids. Modulation of Membrane Trafficking-Genipin/Mrp2 Genipin is an intestinal bacterial metabolite of geniposide, a major ingredient of a herbal medicine, Inchin-ko-to, which have potent choleretic effects, and it rapidly stimulates redistribution of Mrp2 to the canalicular membrane in rats (365). Infusion of genipin for 30 minutes significantly increased the biliary excretion of glutathione in normal rats. Accordingly, genipin treatment increases an insertion of Mrp2 to the canalicular membrane and/or decreases internalization by known mechanism. The same strategy will be also effective in predicting induction of drug transporters. Most transporters involved in the drug disposition are characterized by broad substrate specificities and accept structurally unrelated compounds. Using gene knockout/deficient animals and selective inhibitors, scientists have investigated the roles of transporters in drug disposi- tion. Drug-drug interactions involving transporters include direct inhibition or indirect modulation, and thereby, affect the pharmacokinetics of the substrate drugs. For direct inhibition, using unbound concentration of inhibitors and inhibition constant of the target transporter, one can quantitatively evaluate the degree of inhibition of the target transporter. This rough estimation will be helpful for prescreening of drug-drug interaction and evaluation of in vivo rel- evance of such inhibition in the drug-drug interactions. This chapter focused on the molecular characteristics of drug transporters and drug-drug interaction involving these drug transporters. Inhibition of biliary excretion of methotrexate by probenecid in rats: quantitative prediction of interaction from in vitro data. Quantative prediction of in vivo drug clearance and drug interactions from in vitro data on metabolism together with binding and transport. Prediction of pharmacokinetic alterations caused by drug-drug interactions: metabolic interaction in the liver. Hepatobiliary transport governs overall elimination of peptidic endothelin antagonists in rats. Function of uptake transporters for taurocholate and estradiol 17b - D-glucuronide in cryopreserved human hepatocytes. Evaluation of the uptake of pravastatin by perfused rat liver and primary cultured rat hepatocytes. Formation of extensive canalicular net- works by rat hepatocytes cultured in collagen-sandwich configuration. Correlation of biliary excretion in sandwich- cultured rat hepatocytes and in vivo in rats. Use of Ca modulation to evaluate biliary excretion in sandwich- cultured rat hepatocytes. P-glycoprotein expression, localization, and function in sandwich-cultured primary rat and human hepatocytes: relevance to the hepatobiliary disposition of a model opioid peptide. Characterization of efflux transport of organic anions in a mouse brain capillary endothelial cell line. Transport studies with renal proximal tubular and small intestinal brush border and basolateral membrane vesicles: vesicle hetero- geneity, coexistence of transport system. Characterizing mechanisms of hepatic bile acid transport utilizing isolated membrane vesicles. Preparation of basolateral (sinusoidal) and canalicular plasma membrane vesicles for the study of hepatic transport processes. Mechanisms of taurocholate transport in canalicular and basolateral rat liver plasma membrane vesicles. The function of Gp170, the multidrug resistance gene product, in rat liver canalicular membrane vesicles. Functional involvement of rat organic anion transporter 3 (rOat3; Slc22a8) in the renal uptake of organic anions. The use of sacs of everted small intestine for the study of the transference of substances from the mucosal to serosal surface. Influence of shunt on transmural sodium transport and electrical potential differences. Uptake of the cephalosporin, cephalexin, by a dipeptide transport carrier in the human intestinal cell line, Caco-2. The human intestinal epithelial cell line Caco-2; pharmacological and pharmacokinetic applications. Molecular and functional character- ization of intestinal Na(þ)-dependent neutral amino acid transporter B0. Functional expression of P-glycoprotein in apical membranes of human intestinal Caco-2 cells. Function and expression of multidrug resistance-associated protein family in human colon adenocarcinoma cells (Caco-2). Expression, localization, and functional character- istics of breast cancer resistance protein in Caco-2 cells. Drug absorption limited by P-glycoprotein- mediated secretory drug transport in human intestinal epithelial Caco-2 cell layer. Comparisons of P-glycoprotein expression in isolated rat brain microvessels and in primary cultures of endothelial cells derived from microvasculature of rat brain, epididymal fat pad and from aorta. Multidrug resistance-related trans- port proteins in isolated human brain microvessels and in cells cultured from these isolates. Mrp1 multidrug resistance-associated protein and P-glycoprotein expression in rat brain microvessel endothelial cells. Contribution of sodium taurocholate co- transporting polypeptide to the uptake of its possible substrates into rat hepatocytes. Contribution of organic anion transporting polypeptide to uptake of its possible substrates into rat hepatocytes. Contribution of organic anion trans- porters to the renal uptake of anionic compounds and nucleoside derivatives in rat. Human hepatobiliary transport of organic anions analyzed by quadruple-transfected cells. Organic anion transporting polypeptide 2B1 and breast cancer resistance protein interact in the transepithelial transport of steroid sulfates in human placenta. The renal-specific transporter mediates facilitative transport of organic anions at the brush border membrane of mouse renal tubules. The heteromeric organic solute trans- porter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. Immunologic distribution of an organic anion transport protein in rat liver and kidney. Identification of glutathione as a driving force and leukotriene C4 as a substrate for oatp1, the hepatic sinusoidal organic solute transporter. Cloning and functional characterization of a novel rat organic anion transporter mediating basolateral uptake of methotrexate in the kid- ney.