

William Paterson University. S. Ivan, MD: "Buy online Sumycin cheap no RX - Quality Sumycin OTC".
Stopper the flask and (4) In the case of enriched parboiled swirl it moderately for 1⁄2 minute so rice order sumycin on line antibiotic that starts with c, butylated hydroxytoluene may be that the rice is in motion and in uni- added as an optional ingredient in an form suspension buy generic sumycin online antibiotics for uti co amoxiclav. To the contents of the flask generic 250 mg sumycin antibiotics contagious, section may be added in a harmless add 1,600 milliliters of distilled water carrier. Such carrier is used only in the and 20 milliliters of 10 N hydrochloric quantity necessary to effect an inti- acid. Agitate vigorously and wash mate and uniform mixture of such sub- down the sides of the flask with 150 stances with the rice. In (c) Unless the label of the food bears order to avoid excess foaming during the statement "To retain vitamins do the extraction, heat the mixture slowly not rinse before or drain after cooking" to about 100 °C, agitate if necessary, immediately preceding or following the and maintain at this temperature until name of the food and in letters not less air is expelled. Again wash down the than one-fourth the point size of type sides of the flask with 150 milliliters of used for printing the name of the food 0. Heat the mix- (but in no case less than 8-point type) ture in an autoclave at 120 °C to 123 °C and the label bears no cooking direc- for 30 minutes, remove and cool to tions calling for washing or draining or room temperature. Dilute the mixture unless the food is precooked and it is with distilled water so that the total packaged in consumer packages which volume is 2,500 milliliters. Swirl the are conspicuously and prominently la- flask, and while the solids are in uni- beled with directions for preparation form suspension pour off about 250 mil- which, if followed, will avoid washing liliters of the mixture for later deter- away or draining off enriching ingredi- mination of iron (and calcium, if this is ents, the substances named in para- to be determined). With filter paper graphs (a) (1), (2), and (3) of this section that has been shown not to adsorb thi- shall be present in such quantity or in amine, riboflavin, or niacin, filter such form that when the enriched rice enough of the remaining mixture for is washed as prescribed in paragraph (e) determination of thiamine, riboflavin, of this section, the washed rice con- and niacin. I (4–1–10 Edition) using a suitable analytical filter-aid, individual under customary conditions may be substituted for, or may pre- of purchase. The finished macaroni product con- (3) When the ingredient specified in tains not less than 87 percent of total paragraph (a)(6) of this section is used, solids as determined by the method the label shall bear the statement prescribed in "Official Methods of "Glyceryl monostearate added" or the Analysis of the Association of Official statement "With added glyceryl mono- Analytical Chemists," 13th Ed. Enriched macaroni (b) Enriched macaroni is the enriched products are the class of food each of macaroni product the units of which which conforms to the definition and conform to the specifications of shape standard of identity and is subject to and size prescribed for macaroni by the requirements for label statement of §139. Edible protein dried torula yeast, partly defatted sources, including food grade flours or wheat germ, enriched farina, or en- meals made from nonwheat cereals or riched flour, or through the direct ad- from oilseeds, may be used. Vitamin ditions of any of the substances pre- and mineral enrichment nutrients are scribed in paragraphs (a) (1), (2), and (3) added to bring the food into conformity of this section. Safe and suitable ingre- Iron and calcium may be added only in dients, as provided for in paragraph (c) forms which are harmless and assimi- of this section, may be added. The substances referred to in portion of the milled wheat ingredient paragraphs (a) (1) and (2) of this section is larger than the proportion of any may be added in a harmless carrier other ingredient used. In percent that of casein as determined on lieu of the words "Macaroni Product" the cooked food by the method in sec- the word "Macaroni", "Spaghetti", or tions 43. The enrichment nutrients (3) When, in conformity with para- may be added in a harmless carrier graph (d) (1) or (2) of this section, two used only in a quantity necessary to ef- or more ingredients are listed in the fect a uniform distribution of the nu- name, their designations shall be ar- trients in the finished food. The re- ranged in descending order of predomi- quirements of paragraphs (b) (1) and (2) nance by weight. When the optional ingredient (1)(i) In preparing the dough, nonfat gum gluten (§139. Carrageenan or roni product the units of which con- salts of carrageenan conforming to the form to the specifications of shape and requirements of §172. Iron may be added only in a form that (a) Each of the enriched macaroni is harmless and assimilable. These substances tein derived from the semolina, durum may be added through direct addition flour, farina, flour or any combination or wholly or in part through the use of of these used, does not exceed 13 per- dried yeast, dried torula yeast, partly cent of the weight of the finished food. I (4–1–10 Edition) which conform to the specifications of prescribed for macaroni, spaghetti, or shape and size prescribed for macaroni vermicelli in §139. The blank in each instance is filled in (c) Vegetable spaghetti is the vege- with the name of the vegetable used, as table macaroni product the units of specified in §139. For example, which conform to the specifications of the name of an enriched macaroni shape and size prescribed for spaghetti product containing the prescribed by §139. When the optional ingredient food each of which is prepared by dry- gum gluten (§139. Each of the in- codeloflfederallregulations/ gredients used in the food shall be de- ibrllocations. The total solids of clared on the label as required by the noodle products contains not less than applicable sections of parts 101 and 130 5. The substances referred to in fresh, canned, dried, or in the form of paragraphs (a) (1) and (2) of this section puree or paste). I (4–1–10 Edition) conforms to the definition and stand- fications of shape and size prescribed ard of identity and is subject to the re- for egg macaroni by §139. The blank in each in- is "Wheat and soy noodles", "Wheat stance is filled in with the name of the and soy egg noodles", "Wheat and soy- vegetable used, as specified in bean noodles", "Wheat and soybean §139. Standardized Canned Fruits (e) The term invert sugar sirup means an aqueous solution of inverted or 145. The solids of (j) The term fruit juice(s) means sin- corn sirup and of dried corn sirup con- gle strength expressed juice(s) of tain not less than 40 percent by weight sound, mature fruit(s). It may be fresh, of reducing sugars calculated as anhy- frozen, canned, or made from con- drous dextrose. The bottom of the by such standard prior to the addition sieve is woven-wire cloth which com- of any sweetener which may be used. The avail- (l) The term solid pack means the ability of this incorporation by ref- product contains practically all fruit erence is given in paragraph (m) of this with only the very little free flowing section. Carefully invert by hand all liquid that is expressed from the fruit fruits having cups or cavities if they and to which no packing media have fall on the sieve with cups or cavities been added. Cups or cavities in soft products (m) The procedure for determining may be drained by tilting sieve. With- the densities of the packing media out further shifting the material on means the following: The density of the the sieve, incline the sieve at an angle packing medium, when measured 15 of 17° to 20° to facilitate drainage. Two days or more after packing, or the den- minutes after the drainage begins, sity of the blended homogenized slurry weigh the sieve and drained fruit. The of the comminuted entire contents of weight so found, less the weight of the the container, when measured less than sieve, shall be considered to be the 15 days after canning, is determined ac- weight of the drained fruit. A lot shall for temperature to the equivalent at 20 be deemed to be in compliance for °C, but without correction for invert packing medium density based on the sugar or other substances. A lot shall be (n) The procedure for determining deemed to be in compliance for fill of drained weight is as follows: Tilt the container (packing medium and fruit opened container so as to distribute ingredient) when the number of the contents evenly over the meshes of defectives does not exceed the accept- a circular sieve which has previously ance number (c) in the sampling plans. A container, a por- cific Standardized Canned tion of the contents of a container, or Fruits a composite mixture of product from small containers that is sufficient for §145. Any sample unit shall comminuted or chopped apples (Malus be regarded as defective when the sam- domestica Borkhausen), which may or ple unit does not meet the criteria set may not be peeled and cored, and which forth in the standards. The max- the optional ingredients specified in imum number of defective sample units paragraph (a)(2) of this section. The permitted in the sample in order to apple ingredient is heated and, in ac- consider the lot as meeting the speci- cordance with good manufacturing fied requirements.
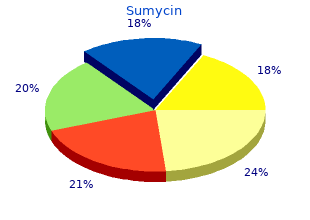
Usage: gtt.
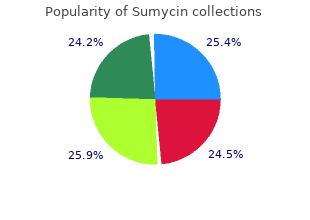
In the Wolff- The antithesis to synthesis Chaikoff effect cheapest sumycin virus mutation rate, excess iodine Thioamides block iodine’s ability to combine with tyrosine discount sumycin 250 mg with amex antibiotics klebsiella, there- decreases the by preventing thyroid hormone synthesis purchase sumycin 500mg mastercard antibiotic youtube. Stable iodine inhibits hormone synthesis through the Wolff- Chaikoff effect, in which excess iodine decreases the formation and release of thyroid hormone. Warning: Radioactive material Radioactive iodine reduces hormone secre- tion by destroying thyroid tissue through in- duction of acute radiation thyroiditis (inflam- mation of the thyroid gland) and chronic gradual thyroid atrophy. Acute radiation thy- roiditis usually occurs 3 to 10 days after ad- ministering radioactive iodine. Pharmacotherapeutics Antithyroid drugs are commonly used to treat hyperthyroidism, especially in the form of Graves’ disease (hyperthyroidism caused by autoimmunity), which accounts for 85% of all cases. In case of removal To treat hyperthyroidism, the thyroid gland may be removed by surgery or destroyed by radiation. Stable iodine is also used after radioactive iodine therapy to control symptoms of hyperthyroidism while the radiation takes effect. Adverse If it gets too severe reactions to Propylthiouracil, which lowers serum T3 levels faster than methi- antithyroid mazole, is usually used for rapid improvement of severe hyperthy- drugs roidism. The most serious ad- When taking them for two verse reaction to Propylthiouracil is preferred over methimazole in pregnant thioamide therapy is women because its rapid action reduces transfer across the pla- granulocytopenia. Hy- cental barrier and it doesn’t cause aplasia cutis (a severe skin dis- persensitivity reactions order) in the fetus. Propylthiouracil and methimazole appear in breast milk, so it’s recommended that mothers taking these drugs shouldn’t breast- In bad taste feed. If a breast-feeding woman must take one of these drugs, The iodides can cause propylthiouracil is the preferred drug. Which signs indicate that a patient taking levothyroxine is ex- periencing thyroid toxicity? Diarrhea and weight loss are signs of thyroid toxicity in a patient taking levothyroxine. Oxytocin stimulates uterine contractions by increas- ing permeability of uterine cell membranes to sodium ions. Drugs and homeostasis Illness can easily disturb the homeostatic mechanisms that help maintain normal fluid and electrolyte balance. Such occurrences as loss of appetite, medication administration, vomiting, diarrhea, Look out! Electrolyte replacement drugs An electrolyte is a compound or element that carries an electrical charge when dissolved in water. Electrolyte replacement drugs are inorganic or organic salts that increase depleted or deficient electrolyte levels, helping to maintain homeostasis, the stability of body fluid composition and volume. Be- cause the body can’t store potassium, adequate amounts must be ingested daily. There, the enzyme adenosinetriphosphatase main- tains the concentration of potassium by pumping sodium out of Because the body the cell in exchange for potassium. It’s an essential element in deter- mining cell membrane potential and excitability. Potassium is necessary for proper functioning of all nerve and muscle cells and for nerve impulse transmission. It’s also essential for tissue growth and repair and for maintenance of acid-base balance. Pharmacotherapeutics (how drugs are used) Potassium replacement therapy corrects hypokalemia, low levels of potassium in the blood. Most adverse reactions Be still my heart to potassium are related Potassium decreases the toxic effects of digoxin. Because potassi- to the method of admin- um inhibits the excitability of the heart, normal potassium levels istration. Oral history Oral potassium some- Drug interactions times causes nausea, Potassium should be used cautiously in patients receiving vomiting, abdominal potassium-sparing diuretics (such as amiloride, spironolactone, pain, and diarrhea. When dietary intake isn’t enough to meet metabolic needs, pain at the injection site calcium stores in bone are reduced. Ionized calcium is the sium in patients with de- physiologically active form and plays a role in cellular functions. Absorbed with absorption Absorption also depends on dietary factors, such as calcium bind- ing to fiber, phytates, and oxalates, and on fatty acids, with which calcium salts form insoluble soaps. Calcium salts are elimi- nated unchanged primarily in stool; the remainder is excreted in urine. Calcium has several important roles in the body: • Extracellular ionized calcium plays an essential role in normal nerve and muscle excitability. It also helps strengthen myocardial tissue after defibrillation (electric shock to restore normal heart rhythm) or a poor response to epi- nephrine during resuscitation. Pregnancy and breast-feeding in- crease calcium requirements, as do periods of bone growth during childhood and adolescence. Chronic hypocalcemia from such conditions as chronic hypoparathyroidism (a deficiency of parathyroid hormones), osteomalacia (softening of bones), long- term glucocorticoid therapy, and plicamycin and vitamin D defi- ciency is also treated with oral calcium. Adverse • When given in total parenteral nutrition, calcium may react with reactions phosphorus present in the solution to form insoluble calcium to calcium phosphate granules, which may find their way into pulmonary ar- terioles, causing emboli and possibly death. Take this to heart Electrocardiogram Traffic control changes that occur with Magnesium also aids in cell metabolism and the movement of elevated serum calcium sodium and potassium across cell membranes. Severe Magnesium stores may be depleted by: hypercalcemia can • malabsorption cause cardiac arrhyth- • chronic diarrhea mias, cardiac arrest, and • prolonged treatment with diuretics coma. Restocking the mineral stores Magnesium is typically replaced in the form of magnesium sulfate when administered I. Metabolism and excretion Magnesium sulfate isn’t metabolized and is excreted unchanged in urine and stool; some appears in breast milk. Pharmacodynamics Magnesium sulfate replenishes and prevents magnesium deficien- cies. It’s widely used to treat or prevent preeclamptic and eclamptic sei- zure activity and is used to treat ventricular arrhythmias such as torsades de pointes. It’s also used to treat seizures, severe toxe- mia, and acute nephritis in children. Diuretics and tap water enemas can also de- plete sodium, particularly when fluids are replaced by plain water. The salt flats Sodium also can be lost in trauma or wound drainage, adrenal When I’m out gland insufficiency, cirrhosis of the liver with ascites, syndrome of jogging, I lose inappropriate antidiuretic hormone, and prolonged I. Sodium can help me replace depleted Calling all chlorides fluids and maintain Sodium is typically replaced in the form of sodium chloride. Pharmacokinetics Oral and parenteral sodium chloride are quickly absorbed and distributed widely throughout the body.
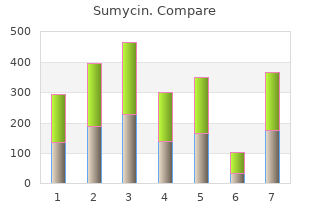
Toluene 50 ml is added with constant shaking until the mixture turns hazy in appearance order sumycin 500 mg overnight delivery antimicrobial index. The process is repeated by the alternate addition of methanol and benzene until 1 litre of solution is obtained generic sumycin 500mg with mastercard virus barrier express, taking care to add a minimum volume of methanol to give a visible clear solution sumycin 250 mg overnight delivery bacteria war. Magnetic stirrer Caution : Care must be taken to avoid contamination of neutralized liquid with atmospheric carbon dioxide. In summing up, the net reaction in the process of standardization has been expressed. Tetrabutylammonium Hydroxide The alkalimetry in non-aqueous titrations may also be carried out efficiently by using tetrabutylammonium hydroxide along with an appropriate indicatior. Centrifuge about 2-3 ml of the resultant mixture and test for iodide in the supernatant liquid. In case, it gives a positive test, add about 2 g more of silver oxide and shake for an additional period of 30 minutes. The said method may be repeated until the supernatant liquid obtained is completely free from iodide. The mixture thus obtained is filtered through a fine sintered glass filter and finally rinse the container with 3 portions, each of 50 ml of dry toluene. These washings may be added to the filtrate and the final volume is made upto 1 litre with dry toluene. Procedure : Accurately weigh about 60 mg of benzoic acid into 10 ml of previously neutralized dimethyl formamide to the blue colour of thymol blue (3 drops) by titration against 0. Cognate Assays The following pharmaceutical substances may be assayed by employing tetrabutylammonium hydroxide either by using a suitable indicator titrimetrically or potentiometrically as given in Table 5. As these are monobasic acids in character, therefore, they react quantitatively in a non- aqueous media with the base titrant, employing typical acid-base indicators to detect the end-points. How does ‘pyridine’–a weak base behaves as a strong base in acetons-perchloric acid? Why is it advised to keep the solution overnight before carrying out the actual assay with it? Based on ‘acidimetry in non-aqueous titrations’, how do we carry out the assay of the following ‘drugs’ along with their theory, procedure and calculations : (i) Methyldopa ; (ii) Adrenaline (iii) Metronitazole ; (iv) Salbutamol sulphate. How would you perform the non-aqueous titrations of pure drugs or their dosage forms petentiometrically? Explain in details the acidimetric assays of the following ‘drugs’ : (i) Diazepam (ii) Mebendazole (iii) Physostigmine Injection (iv) Trimethoprim. How would your assay Niclosamide and Chlorthalidone using tetrabutyl-ammonium hydroxide either potentiometrically or titrimetrically by non-aqueous titrations. Safarik, ‘Titrations in Non-Aqueous Solvents’, New York, Elsevier North-Holland, 1965. In the oxidation—reduction methods of analysis a change in valence of the reacting products is a must which is contrary to precipitation and neutralization methods of analysis where no change in valence occur. The major oxidizing agents normally employed in volumetric titrations include, potassium permanganate, potassium dichromate, and ceric sulphate. It is, therefore, pertinent to observe here that 2 whenever one entity undergoes oxidation, definitely some other entity undergoes reduction correspondingly and vice-versa. In other words, there always exists a transfer of electrons in oxidation-reduction reactions, because in every such reaction the charge gained or lost by one substance must essentially be lost or gained by another. A reducing agent is the reactant that loses electrons in an oxidation-reduction reaction : Fe2+ → Fe3+ +e Ce3+ → Ce4+ +e Thus, the reactant containing a constituent atom or atoms are converted to a higher state of oxidation. An oxidizing agent is the reactant that gains electrons in an oxidation-reduction reaction : Ce4+ +e– → Ce3+ Fe3+ +e– → Fe2+ Thus, the reactant containing a constituent atom or atoms are converted to a lower state of oxidation. The quantitative measurement of one of the reactants may be accomplished by the reaction derived from the combination of oxidizing and reducing agents, for instance Fe2+ +Ce4+ → Fe3+ + Ce3+ and hence, ferrous sulphate can be estimated quantitatively by its reaction with ceric sulphate. Transfer the contents to a 250 ml beaker containing cold water and stir vigorously with a glass rod to effect rapid dissolution. Decant the solution through a small plug of glass wool supported by a funnel, into a 1 litre volumetric flask thereby leaving the undissolved residues in the beaker. Finally make up the volume to the graduated mark and shake well so as to effect uniform mixing. Pipette out 25 ml of this solution, add to it 5 ml of concentrated sulphuric acid along the side of the flask, swirl the contents carefully and warm upto 70°C. Titrate this against the potassium permanganate solution from the burette till the pink colour persists for about 20 seconds. Direct Titration Methods Hydrogen peroxide solution and potassium bromide are two pharmaceutical substances that may be estimated by employing 0. Hydrogen Peroxide Solution Materials Required : Hydrogen peroxide solution : 10 ml ; 5 N sulphuric acid : 5 ml ; 0. Hence, decompo- sition takes place as designated by the following equation : 2H2O2 → 2H2O + O2 or 68. Indirect Titration Methods In the indirect method of permanganate oxidation certain compounds are first converted by means of chemical reactions to an equivalent amount of oxalate which is then subsequently oxidized quantitatively by permanganate. Assay of Cherry Juice for Malic Acid In this particular assay the malic acid present in the cherry juice is estimated by the following three steps sequentially : Step 1 : Conversion of malic acid to an equivalent amount of calcium salt, Step 2 : Conversion of calcium salt to corresponding insoluble calcium oxalate, and Step 3 : Liberation of oxalate and subsequent oxidation with permanganate. Procedure : Place 10 ml of precisely measured cherry juice in a 125 ml flask and add to it 1 g of calcium carbonate. Heat the contents on a water-bath for 15 minutes while swirling periodically and filter. Potassium dichromate possesses an inherent oranage colour that is not intense enough to serve its own end-point signal, specifically in the presence of the green Cr3+ ion, which is supposed to be present at the end-point. Note : Potassium dichromate can be obtained as a primary standard reagent and hence, standard solu- tions may be prepared determinately and stored for long periods of time. Calculations : The quantity of Mohr’s salt required for 250 ml of the solution having a normality of 0. Procedure : Transfer 20 ml of the primary standard solution (Mohr’s salt) to the titration flask and add 20 ml of 2 N sulphuric acid. Transfer drops of the titrated solution by means of a glass rod and mix with drops of the indicator, already taken in the groove-tile. The above sequential steps give fairly accurate results because the error caused by the removal of part of the solution for the spot tests is made negligibly small. By applying the relationship between N1V1 (K2Cr2O7) and N2V2 (Mohr’s salt), the normality of the former may be calculated. Procedure : (a) Preparation of Standard K2Cr2O7 Solution : Instead of using solutions having definite normal- ity, routine industrial laboratories make use of ‘emperical solution’ which is normally expressed in terms of ‘titer for the substance determined’. For this assay, let us prepare an emperical K2Cr2O7 solution (250 ml) of such a concentration that 1 ml of the same exactly correspond to 0. Add 15 ml of concen- trated hydrochloric acid, warm the contents of the flask carefully over a sand-bath until most of the dark grains of ore get dissolved completely and only a whitish silica precipitate settles at the bottom of the flask.