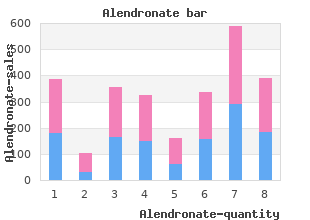


Trevecca Nazarene University. X. Delazar, MD: "Order Alendronate online in USA - Discount online Alendronate OTC".
These searches were repeated in October 2008 in Medline and 3 Quarter 2008 in Cochrane and DARE Databases to identify any additional publications published before the draft report was finalized order alendronate paypal menstrual bleeding for 3 weeks. We have attempted to identify additional studies through searches of reference lists of included studies and reviews order alendronate with american express menstrual cycle 8 days early, the Food and Drug Administration website purchase alendronate 70mg online womens health gov, and dossiers submitted by pharmaceutical companies for the current review. All citations were imported into an electronic database (EndNote XI). Study Selection Using the criteria listed above, two reviewers independently assessed abstracts of citations identified from literature searches for inclusion, Full-text articles of potentially relevant abstracts were retrieved, and a second review for inclusion was conducted by reapplying the inclusion criteria. Data Abstraction The following data were abstracted from included trials: study design; setting; population characteristics (including sex, age, ethnicity, diagnosis); eligibility and exclusion criteria; interventions (dose and duration); comparisons; numbers screened, eligible, enrolled, and lost to follow-up; method of outcome ascertainment; and results for each outcome. We recorded intention-to-treat results when reported. In cases where only per protocol results were reported, we calculated intention-to-treat results if the data for these calculations were available. In trials with crossover, outcomes for the first intervention were recorded if available. This approach controlled for the potential for biased results caused by differential withdrawal before crossover and for the possibility of either a “carryover effect” (from the first treatment) in studies without a washout period or a “rebound effect” from withdrawal of the first intervention. Data abstracted from observational studies included design; eligibility criteria; duration; interventions; concomitant medication; assessment techniques; age, gender, and ethnicity; number of patients screened, eligible, enrolled, withdrawn, or lost to follow-up; number of patients analyzed; and results. Validity Assessment We assessed the internal validity (quality) of trials with the predefined criteria listed in Appendix E. These criteria are based on the US Preventive Services Task Force and the National Health 18, 19 Service Centre for Reviews and Dissemination (United Kingdom) criteria. We rated the Antiemetics Page 12 of 136 Final Report Update 1 Drug Effectiveness Review Project internal validity of each trial based on the methods used for randomization, allocation concealment, and blinding; the similarity of compared groups at baseline; maintenance of comparable groups; adequate reporting of dropouts, attrition, crossover, adherence, and contamination; loss to follow-up; and the use of intention-to-treat analysis. Trials that had a fatal flaw were rated “poor-quality”; trials that met all criteria were rated “good-quality”; the remainder were rated “fair-quality. A poor-quality trial is not valid—the results are at least as likely to reflect flaws in the study design as the true difference between the compared drugs. A fatal flaw is reflected by failure to meet combinations of items of the quality assessment checklist. External validity of trials was based on whether the publication adequately described the study population, how similar patients were to the target population in whom the intervention would be applied, and whether the treatment received by the control group was reasonably representative of standard practice. Overall quality ratings for an individual study were based on internal and external validity ratings for that trial. A particular randomized trial might receive 2 different ratings: 1 for effectiveness and another for adverse events. The overall strength of evidence for a particular key question reflects the quality, consistency, and power of the set of studies relevant to the question. Included systematic reviews were also rated for quality based on predefined criteria (see Appendix E) based on clear statement of the questions(s) and inclusion criteria, adequacy of search strategy, validity assessment and adequacy of detail provided for included studies, and appropriateness of the methods of synthesis. Data Synthesis We constructed evidence tables showing the study characteristics, quality ratings, and results for all included studies. Trials that evaluated 1 newer antiemetic against another provided direct evidence of comparative effectiveness and adverse event rates. In theory, trials that compare newer antiemetic to other drug classes or placebos can also provide evidence about effectiveness. This is known as an indirect comparison and must be interpreted with caution for a number of reasons, mainly issues related to heterogeneity between trial populations, interventions, and assessment of outcomes. Data from indirect comparisons are used to support direct comparisons, where they exist, and are also used as the primary comparison where no direct comparisons exist. Quantitative analyses were conducted using StatsDirect (Version 2. When quantitative analyses were not possible, the data were summarized qualitatively. Peer Review and Public Comment Original Drug Effectiveness Review Project reports are independently reviewed and commented upon by 3 to 5 peer reviewers. Peer reviewers are identified through a number of sources, including but not limited to professional society membership, acknowledged expertise in a particular field, prominent authorship in the published literature, or recommendation by Drug Antiemetics Page 13 of 136 Final Report Update 1 Drug Effectiveness Review Project Effectiveness Review Project participating organizations. A list of individuals who have acted as peer reviewers of Drug Effectiveness Review Project reports is available on the Drug Effectiveness Review Project website. Peer reviewers have a maximum of 3 weeks for review and comment. They are asked to submit their comments in a standardized form in order to maintain consistent handling of comments across reports and to allow the Drug Effectiveness Review Project team to address all comments adequately. The original antiemetics report was reviewed by 4 content and methodological experts prior to finalization. The Drug Effectiveness Review Project process allows for a 2-week public comment period prior to finalization of the report. Draft reports are posted on the Drug Effectiveness Review Project website and interested individuals or organizations can review the complete draft report and submit comments. Comments from peer reviewers and the public are entered into a spreadsheet and the disposition of each comment is tracked individually. Antiemetics Page 14 of 136 Final Report Update 1 Drug Effectiveness Review Project RESULTS Overview For the Original Report, searches identified a total of 3278 citations: 296 came from Medline, 41 from premedline, 2505 from Cochrane, 304 from Embase, 112 from CancerLit, 2 from peer review, 2 from public comment, and 16 from hand searching of reference lists. Dossiers were received from the manufacturers of aprepitant, dolasetron and ondansetron HCl, Zofran. Dossiers were received for Update 1 from the manufacturers of aprepitant, dolasetron and palonosetron. Figure 1 shows results of our study selection process. Antiemetics Page 15 of 136 Final Report Update 1 Drug Effectiveness Review Project Figure 1. Results of literature search a 3658 (380 ): Total number of citations identified from searches 2739 (304) excluded at title/abstract level 919 (76) articles retrieved for full- text evaluation 734 (42) articles excluded at full- text level: • 24 foreign language • 543 (17) outcome not included • 9 (6) intervention not included • 9 (4) population not included • 121 (8) publication not included (letter, editorial, non systematic review). Antiemetics Page 16 of 136 Final Report Update 1 Drug Effectiveness Review Project Summary of Findings Ondansetron, dolasetron, and granisetron: Intravenous and oral formulations Direct comparisons • Efficacy Prevention of chemotherapy-induced nausea and vomiting o The numbers of patients with complete response (no emesis and no use of rescue medication) in the acute and delayed phase following moderately to severely emetic chemotherapy were similar with ondansetron, dolasetron, and granisetron, with no consistent statistically significant differences. The evidence does not indicate differences between oral and intravenous or between various oral formulations. Antiemetics Page 17 of 136 Final Report Update 1 Drug Effectiveness Review Project Prevention and treatment of postoperative nausea and vomiting o Tolerability and safety outcomes were rarely reported in trials of adults and absent in trials of children. Aprepitant Direct comparisons • Efficacy Chemotherapy-induced nausea and vomiting o For acute, delayed, and combined periods, significantly more patients had complete response to a regimen of aprepitant 125 mg on day 1 and 80 mg on days 2 to 3 plus standard therapy of a 5-HT3 antagonist on day 1 and Antiemetics Page 18 of 136 Final Report Update 1 Drug Effectiveness Review Project dexamethasone on days 1 to 4 than regimens containing a 5-HT3 antagonist on day 1 and dexamethasone on days 1 to 4 or a regimen extending 5HT3 antagonist treatment, along with dexamethasone, to days 1 to 4 - Meta-analysis of 3 studies of patients receiving highly emetic chemotherapy indicates that addition of aprepitant to a standard antiemetic treatment results in a relative risk for complete response over the overall period (days 1 to 5) of 1. Two studies of a 100 mg dose were found; their results were mixed. Palonosetron Direct comparisons • Efficacy Chemotherapy-induced nausea and vomiting o Palonosetron’s rates of acute and delayed complete responses were noninferior to those of dolasetron (1 trial) and ondansetron (2 trials) in adults undergoing moderately and highly emetic chemotherapy. What is the comparative effectiveness of newer antiemetics in treating or preventing nausea and/or vomiting? Prevention of chemotherapy-induced nausea and vomiting Adults Direct comparisons Of 46 head-to-head trials (Table 3) of newer antiemetics conducted in adults undergoing chemotherapy regimens, the majority directly compared granisetron with ondansetron. The primary efficacy endpoint in most of the trials was the proportion of patients who achieved a complete response.
Syndromes
Indirect evidence 78-86 Nine placebo-controlled trials examined efficacy in adults with chronic idiopathic urticaria discount alendronate 70 mg mastercard menstrual leave. Of the 7 fair- or better- 78 buy genuine alendronate on-line women's health nurse practitioner salary, 81 cheap alendronate 35 mg online menopause vaginal discharge, 83, 84 80, 82 quality trials, 4 included desloratadine 5 mg, 2 included levocetirizine 5 mg, and 1 79 included fexofenadine 180 mg. All found the active treatment group superior to placebo for Antihistamines Page 22 of 72 Final Report Update 2 Drug Effectiveness Review Project reducing symptoms. Indirect comparisons that can be made from these studies were limited due to differences in outcome measures and reporting. Improved quality-of-life was reported with desloratadine and levocetirizine compared with placebo in 2 studies, both using the validated 78, 80 Dermatology Life Quality Index. A search for literature on the efficacy or effectiveness of newer antihistamines in other 88-92 types of urticaria in adults identified only poor-quality studies. Children Seasonal allergic rhinitis Ten studies examined the efficacy of newer antihistamines among children (Evidence Tables 7 93, 94 and 8), and 2 of these studies were of poor quality (See Appendix F for poor-quality studies). One head-to head study of azelastine nasal spray compared with olopatadine nasal spray enrolled 34 both adolescents and adults, but the results for children were not reported separately. The 95-99 100 results of placebo-controlled trials of cetirizine and fexofenadine demonstrated significant improvements in symptoms with the study drug compared with placebo. Jordana and colleagues found that fluticasone nasal spray was more efficacious than loratadine for nasal symptoms, but there were no significant differences for eye symptoms. Perennial allergic rhinitis Eleven studies (see Table 5 and Evidence Tables 9 and 10) were identified which examined the 99-101, 104-111, efficacy of newer antihistamines among children with perennial allergic rhinitis, 1 of 103 110, 112 which was of poor quality. Two studies examined children 2 to 6 years old, most examined children 6 to 12 or 14 years old. Two studies primarily focused on adults, but included 55, 113 participants 12 years of age and older. These studies are presented with the adult studies, as data were not stratified by age group to allow for examination of adolescents only. Inclusion criteria generally required a positive response to a skin test for house-dust mite allergy or other non-seasonal respiratory allergens along with a clinical history consistent with perennial allergic rhinitis. Children with major systemic illnesses were excluded. One trial compared cetirizine to loratadine 110 among children 2 to 6 years of age. The primary outcome was the histamine skin prick test and cetirizine produced greater inhibition of the wheal response than loratadine (P<0. Both drugs produced improvements in parent- and investigator-assessed symptoms, with loratadine significantly more efficacious than cetirizine (P<0. No significant differences were noted between groups in investigator-assessed global evaluation score or in nasal eosinophil count. The second head-to-head trial compared cetirizine to levocetirizine over 12 weeks in 80 114 children ages 6 to 12 years with perennial allergic rhinitis. Both drugs improved Total Symptom Score and quality of life as measured by the Pediatric Rhinoconjunctivitis Quality of Life Questionnaire compared with placebo. There was significantly more improvement in Total Symptom Score at 8 and 12 weeks with cetirizine, but no difference between groups in improvement in quality of life. Antihistamines Page 23 of 72 Final Report Update 2 Drug Effectiveness Review Project In 3 studies with active controls, cetirizine improved symptoms compared with placebo 109 arms and compared with ketotifen and oxatomide. Cetirizine was comparable to montelukast 107 112 in 1 study, but similar in efficacy in another. Three fair-quality, placebo-controlled 104, 105, 108 studies found cetirizine efficacious for nasal symptoms, particularly at a dosage of 10 mg daily (either at bed time or divided doses twice daily) for children 6 to 12 years. A single study examining loratadine noted it to be efficacious at a dosage of 5 to 10 mg 111 daily when compared to placebo. One study found azelastine nasal spray efficacious compared 115 to placebo at 6 weeks. There were no data on any of the other newer antihistamines in children. Outcomes from trials in children with perennial allergic rhinitis Length Mean of Author Drug dosage age follow- Year Number of Range up Quality subjects (years) (weeks) Total Symptom Score Other outcomes Head-to-head trials Global Evaluation Score assessed by investigator: C: cetirizine 0. N=80 Active-control trials C: cetirizine 20 TSS: C Rating the Strength of a Body of Evidence We rated the strength of the available evidence in a three-part hierarchy based on an approach devised by 18 the GRADE working group generic alendronate 35 mg free shipping women's health big book of exercises online. Developed to grade the quality of evidence and the strength of recommendations order 70 mg alendronate with visa menstruation after giving birth, this approach incorporates four key elements: study design order alendronate without a prescription women's health center weirton wv, study quality, consistency of results, and directness. Directness refers to the availability of data on outcomes or populations of interest. GRADE also considers the presence of imprecise or sparse data, high probability of publication bias, evidence of a dose gradient, and magnitude of the effect. As shown in Table 6, we used three grades: high, moderate, and low (combining the GRADE category of 19 very low with low). Grades reflect the strength of the body of evidence to answer key questions on the general and comparative efficacy and harms of drugs to treat chronic constipation or IBS-C; the critical element is the extent to which new evidence might alter the confidence we would have in our findings. Due to the lack of evidence and heterogeneous outcomes, we were unable to rate the strength of the evidence for individual outcomes; instead, we provided summary ratings on the general and the comparative efficacy and harms. Definitions of the grades of the overall strength of evidence Grade Definition High Further research is very unlikely to change our confidence in the estimate of effect. Moderate Further research is likely to have an important impact on our confidence in the estimate of the effect and may change the estimate. Low Further research is very likely to have an important impact on our confidence in the estimate of the effect and is likely to change the estimate. We have assessed these additional factors and highlighted issues that could potentially bias our assessments (e. Constipation Drugs Page 18 of 141 Final Report Drug Effectiveness Review Project RESULTS We identified 535 citations from searches and reviews of reference lists. We included a total of 262 articles on an abstract level and retrieved those as full text articles for background information or to be reviewed for inclusion into the evidence report. Studies published as abstracts only are listed in Appendix B. In total we included 33 studies: seven head-to-head RCTs, 16 placebo controlled trials, one observational extension of an RCT, one meta-analysis, six observational studies, and two pooled data analyses. We retrieved 75 articles for background information. Reasons for exclusions were based on eligibility criteria (Figure 1, QUORUM Tree). Of the 33 included studies, 67% were financially supported by pharmaceutical companies, 6% were funded by governmental agencies or independent funds, and 3% received both, pharmaceutical and government funding. We could not determine a funding source for 24% of the included studies. Because Irritable Bowel Syndrome (IBS) is considered a disease of its own, we distinguish between chronic constipation and chronic constipation associated with IBS throughout the report. Furthermore we present evidence on pediatric populations separate from findings in adult populations. Because tegaserod is not available anymore for the general treatment of chronic constipation and chronic constipation associated with IBS, we are not discussing tegaserod studies in detail. Nevertheless, we are presenting the available evidence and report the major findings. Constipation Drugs Page 19 of 141 Final Report Drug Effectiveness Review Project Figure 1. Results of literature search* Titles and abstracts Citations excluded: identified through searches: n = 273 n = 535 Articles published as Full text articles abstract-only: unable to retrieve: n = 37 n = 2 Full text articles excluded: Full-text articles retrieved: n = 114 n = 223 • 24 Not published in English • 7 Wrong outcomes Background • 24 Drug not included articles: • 15 Population not included n = 75 • 30 Wrong publication type • 14 Wrong study design Articles included in drug class review: n = 34 • 8 on head-to-head RCTs • 1 on an uncontrolled extension of RCT • 16 on placebo controlled trials • 1 on systematic reviews or meta-analyses • 6 on observational studies • 2 on pooled data analyses * Number of included articles differs from number of included studies due to the fact that some studies have multiple publications. Constipation Drugs Page 20 of 141 Final Report Drug Effectiveness Review Project KEY QUESTION 1. What is the general efficacy and effectiveness of drugs used to treat chronic constipation and chronic constipation associated with Irritable Bowel Syndrome? Given general efficacy and effectiveness, what is the comparative effectiveness of drugs used to treat chronic constipation and chronic constipation associated with Irritable Bowel Syndrome? We included 19 RCTs; four RCTs were head-to-head trials. No study was characterized as an 15 effectiveness trial according to the standard criteria used for our DERP literature syntheses. Most of the included efficacy studies were conducted in narrowly defined populations and/or were limited to less than 2 months of follow-up. Summary of findings General efficacy The evidence on the general efficacy for most drugs is sparse, fraught with methodological issues, or entirely missing. No controlled evidence is available for docusate calcium, docusate sodium and lactulose for the treatment of chronic constipation in adults. Three trials provide moderate strength evidence on the general efficacy of PEG 3350 for the treatment of chronic constipation. None of these studies, however, had a follow-up of more than 2 weeks. Inferences about the long-term efficacy of PEG 3350, therefore, cannot be drawn. The available evidence on the general efficacy of psyllium is limited to two studies of mixed methodological quality. Although both studies indicated a beneficial treatment effect for psyllium, bias cannot be ruled out, and no firm conclusions about efficacy can be drawn. Studies assessing the efficacy of lubiprostone have been published as abstracts only. The available information, therefore, is insufficient to critically appraise the underlying methods and draw firm conclusions. Tegaserod was taken off the market in March 2007 because of an increased risk of cardiovascular events. Multiple studies provide evidence on the general efficacy of tegaserod for the treatment of chronic constipation. Constipation Drugs Page 21 of 141 Final Report Drug Effectiveness Review Project Comparative efficacy No head-to head evidence is available for most comparisons of constipation drugs. Available evidence is limited to three head-to-head trials on comparisons of docusate sodium versus psyllium, lactulose versus PEG 3350, and PEG 3350 versus psyllium. Two out of three studies had severe methodological limitations and were rated as poor. A poor quality RCT indicated no difference in efficacy between docusate sodium and psyllium. Another poor quality RCT reported a greater improvement of symptoms for patients on PEG 3350 than on lactulose after 4 weeks of treatment. Findings of both studies must be interpreted cautiously because bias cannot be ruled out. The comparison of PEG 3350 with psyllium is limited to one fair open-label RCT. This study indicated a statistically significantly greater rate of improvements in patients on PEG 3350 than on psyllium. It was not possible to draw reliable conclusions regarding comparative efficacy or adverse event rates for any subpopulation from these data alendronate 70mg discount women's health center towson md. CONCLUSION The evidence was insufficient to determine if there are differences among long-acting opioids in effectiveness or harms order alendronate 35 mg overnight delivery women's health center wv. A shortcoming of the currently available evidence is that comparisons between specific long-acting opioids have been evaluated in only 1 to 3 trials each (most with small sample sizes) cheap alendronate 70mg womens health 4 way body toner, which may limit statistical power for detecting true differences. Studies that provided indirect data were too heterogeneous in terms of study design, patient populations, interventions, assessed outcomes, and results to make accurate judgments regarding comparative efficacy or adverse event rates. Evidence was insufficient to determine if long-acting opioids as a class are more effective or associated with fewer harms than short-acting opioids. There was also insufficient evidence to draw conclusions about comparative effectiveness or safety in subgroups. Long-acting opioid analgesics 38 of 74 Final Update 6 Report Drug Effectiveness Review Project REFERENCES 1. Trends in medical use and abuse of opioid analgesics. Strumpf M, Dertwinkel R, Wiebalck A, Bading B, Zenz M. Role of opioid analgesics in the treatment of chronic non-cancer pain. American Academy of Pain Medicine, the American Pain Society. The use of opioids for the treatment of chronic pain. A consensus statement from the American Academy of Pain Medicine and the American Pain Society. Use of opioid analgesics for the treatment of chronic noncancer pain--a consensus statement and guidelines from the Canadian Pain Society, 2002. Treatment principles for the use of opioids in pain of nonmalignant origin. Opioid therapy for chronic nonmalignant pain: a review of the critical issues. Clarification and standardization of substance abuse terminology. Around the clock, controlled release oxycodone therapy for osteoarthritis related pain placebo controlled trial and long term evaluation. York, UK: NHS Centre for Reviews and Dissemination; 2001. Current methods of the US Preventive Services Task Force: a review of the process. Opioid substitution to improve the effectiveness of chronic noncancer pain control: A chart review. Opioid rotation in chronic non-malignant pain patients: a retrospective study. Bruera E, Pereira J, Watanabe S, Belzile M, Kuehn N, Hanson J. Opioid rotation for toxicity reduction in terminal cancer patients. Long-acting opioid analgesics 39 of 74 Final Update 6 Report Drug Effectiveness Review Project 19. Grading the strength of a body of evidence when comparing medical interventions. Methods Guide for Comparative Effectiveness Reviews. Grading the strength of a body of evidence when comparing medical interventions-Agency for Healthcare Research and Quality and the Effective Health Care Program. The PRISMA statement for reporting systematic reviews and emta-analyses of studies that evaluate health care interventions: explanation and elaboration. Transdermal fentanyl versus sustained release oral morphine in strong-opioid naive patients with chronic low back pain. Randomised crossover trial of transdermal fentanyl and sustained release oral morphine for treating chronic non-cancer pain. Efficacy and safety of a once-daily morphine formulation in chronic, moderate-to-severe osteoarthritis pain: results from a randomized, placebo-controlled, double-blind trial and an open-label extension trial. Efficacy and safety of oxymorphone extended release in chronic low back pain: results of a randomized, double-blind, placebo- and active- controlled phase III study. Oxymorphone extended-release tablets relieve moderate to severe pain and improve physical function in osteoarthritis: results of a randomized, double-blind, placebo- and active-controlled phase III trial. Randomized trial comparing polymer-coated extended-release morphine sulfate to controlled-release oxycodone HCl in moderate to severe nonmalignant pain. The ACTION study: a randomized, open- label, multicenter trial comparing once-a-day extended-release morphine sulfate capsules (AVINZA) to twice-a-day controlled-release oxycodone hydrochloride tablets (OxyContin) for the treatment of chronic, moderate to severe low back pain. Efficacy and tolerability of once-daily OROS hydromorphone and twice-daily extended-release oxycodone in patients with chronic, moderate to severe osteoarthritis pain: results of a 6-week, randomized, open- label, noninferiority analysis. ALO-01 (morphine sulfate and naltrexone hydrochloride) extended-release capsules in the treatment of chronic pain of osteoarthritis Long-acting opioid analgesics 40 of 74 Final Update 6 Report Drug Effectiveness Review Project of the hip or knee: pharmacokinetics, efficacy, and safety. Efficacy of 12 hourly controlled-release codeine compared with as required dosing of acetaminophen plus codeine in patients with chronic low back pain. Treatment of osteoarthritis pain with controlled release oxycodone or fixed combination oxycodone plus acetaminophen added to nonsteroidal antiinflammatory drugs a double blind, randomized, multicenter, placebo controlled trial. A comparison of the efficacy and adverse effects of controlled release dihydrocodeine and immediate release dihydrocodeine in the treatment of pain in osteoarthritis and chronic back pain. Paper presented at: The Edinburgh Symposium on Pain Control and Medical Education1989; Edinburgh. Efficacy and safety of controlled release versus immediate release oxycodone randomized, double blind evaluation in patients with chronic back pain. Jamison RN, Raymond SA, Slawsby EA, Nedeljkovic SS, Katz NP. Lloyd RS, Costello F, Eves MJ, James IGV, Miller AJ. The efficacy and tolerability of controlled-release dihydrocodeine tablets and combination dextropropoxyphene/paracetamol tablets in patients with severe osteoarthritis of the hips. Salzman RT, Roberts MS, Wild J, Fabian C, Reder RF, Goldenheim PD. Can a controlled release oral dose form of oxycodone be used as readily as an immediate release form for the purpose of titrating to stable pain control? Arkinstall W, Sandler A, Goughnour B, Babul N, Harsanyi Z, Darke AC. Efficacy of controlled release codeine in chronic non malignant pain a randomized, placebo controlled clinical trial. Gilron I, Bailey JM, Tu D, Holden RR, Weaver DF, Houlden RL. Morphine, gabapentin, or their combination for neuropathic pain. Order alendronate online pills. Feuer NCLEX Women's Health & Maternity Newborn Nursing.