

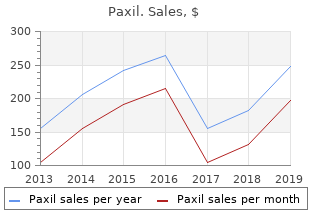
Webster University. Z. Trompok, MD: "Order online Paxil cheap no RX - Effective online Paxil no RX".
Three specific areas of concern with antimuscarinic medication deserve special mention: urinary retention generic 10mg paxil with mastercard symptoms ulcerative colitis, cognitive impairment discount paxil express pretreatment, and glaucoma 10mg paxil fast delivery medicine 666 colds. In the past, there was a universal concern regarding the risk of urinary retention when prescribing antimuscarinic drugs. If this did not occur, urinary retention would result from inability of the bladder to contract. More recently, a concern over the association of anticholinergics and cognitive impairment has prompted several studies evaluating reaction time, memory, confusion, and other cognitive decrements. In a longitudinal cohort study involving 372 adults age >60 years without dementia at recruitment, the effects of continuous anticholinergic drug use on cognition were assessed [104]. In this study, 80% of anticholinergic users were classified as having mild cognitive impairment compared with only 35% of nonusers. There was no difference between users and nonusers in the risk of developing dementia after 8 years of follow-up. Other studies in continent elderly volunteers have shown no significant effects on cognition [27]. However, cholinesterase inhibitors that are often used to improve cognition in Alzheimer’s disease have been shown to precipitate urinary incontinence [105]. Both conditions increase in prevalence with age, and it has been estimated that the conditions coexist in approximately 11. The distinction between open-angle and narrow-angle glaucoma is an important one, and when the answer is unknown, referral to an ophthalmologist is imperative. In the remaining 25% with narrow-angle glaucoma, the risk was felt to be elevated only if iridotomy has not been performed or has not successfully controlled the disease, reducing the true contraindication rate to approximately 8. Interestingly, the same study found that 33% of the patients did not report glaucoma on their medical intake form. Complaints of eye pain, headache, or visual loss following initiation of anticholinergic therapy should be taken seriously and prompt medical advice should be sought [107]. Calcium Antagonists Calcium channels and free intracellular calcium play a large role in excitation–contraction coupling in striated, cardiac, and smooth muscle [108]. These drugs are contraindicated in patients with poor cardiac conduction and those taking β-blockers [110]. It has also been shown to completely block the noncholinergic portion of contraction in the rabbit bladder [112]. These data suggest a potential role for nifedipine in controlling “atropine resistant” contractions, possibly in combination with an antimuscarinic agent. There was no significant difference in the number of incontinent episodes or scores on validated questionnaires in those patients on treatment versus placebo. There is no agent that will specifically block intracellular calcium release only in the bladder smooth muscle cells. Presently, there is no clinical evidence to support the use of calcium-channel inhibitors in the treatment of bladder dysfunction. Potassium-Channel Openers Similar to calcium channels, potassium channels also contribute to the membrane potential of smooth muscle cells and as a result affect smooth muscle tone. Potassium-channel openers relax various types of smooth muscle, including the detrusor, by increasing potassium efflux [79]. Potassium-channel openers are not specific for the bladder, however, and are more potent in relaxing other tissues. In particular, potassium channels are expressed in vascular smooth muscle producing side effects on cardiovascular function, with potentially significant lowering of blood pressure [116]. Common side effects of this class of drug include headaches, flushing, reflex tachycardia, edema, angina, and hypertrichosis [117]. Of the 16 patients, 6 (38%) of them showed a decrease in micturition frequency and an increase in volume voided. Long-term observation was not possible because the drug was withdrawn from the market owing to reported adverse effects of high doses in animal toxicology studies. An increase in the duration of detrusor contraction was seen but no other effects on urodynamic parameters were appreciated. A significant drop in blood pressure was seen precluding studies at a higher dose. Pinacidil Pinacidil is a potassium-channel opener that inhibits spontaneous myogenic contractions as well as contractile responses induced by electrical field stimulation and carbachol in isolated human detrusor [120]. A significant decrease in standing blood pressure with stable heart rate was reported. The results showed no improvement in voided volume, micturition frequency, or incontinence episodes. Common side effects include hypertension, headache, diarrhea, dyspepsia, nausea, gastroesophageal reflux, and arthralgias. These drugs are contraindicated in renal impairment, advanced liver disease, and congestive heart failure and in the perioperative setting following coronary artery bypass surgery [124]. There was a 43% incidence of side effects, primarily nausea, vomiting, headache, and gastrointestinal symptoms. Results showed symptomatic relief in daytime micturition frequency and nocturia in the indomethacin group. The incidence of side effects was high, occurring in 19 of 32 patients; no patients withdrew from the study. In vitro studies show a strong dose-related relaxant effect of β -agonists on the bladder body of2 rabbits but little effect on the bladder base or proximal urethra. In isolated human detrusor muscle, the selective β -agonists solabegron and mirabegron were both found to mediate3 muscle relaxation [130,131]. Facilitation of bladder storage with these agents is thought to be the result of both direct inhibition on the detrusor muscle and inhibition of sensory afferents from the bladder. Reported side effects include tachycardia, hypertension, headache, gastrointestinal effects, nervousness, palpitations, elevated serum glucose and lactate, and decreased serum potassium and calcium. These drugs are contraindicated in patients with uncontrolled hypertension and cardiac arrhythmias associated with tachycardia [128]. The drug is rapidly absorbed after oral administration and 55% is excreted unchanged in the urine and 34% excreted unchanged in the feces. Moderate renal impairment and mild hepatic impairment have little impact on drug metabolism and are not clinically important [308]. The treatment groups received either 100 or 150 mg twice daily and experienced a significant reduction in micturition frequency, incontinence episodes, and urgency symptoms, as well as an increase in volume voided. The drug was well tolerated in this study with the most common side effects being headache and gastrointestinal effects. Dose-dependent improvements in micturition frequency and volume voided were noted. After 12 weeks, both treatment groups demonstrated a statistically significant decrease in micturition frequency and incontinence episodes compared to placebo.
Diseases
These reflexes require the integrative action of neuronal populations at various levels of the neuraxis (Figure 23 discount paxil 20mg with visa treatment bacterial vaginosis. Complete bladder emptying is facilitated by urethra to bladder reflex occurring while urine is flowing through urethra as demonstrated in anesthetized cat experiments by Barrington [72 buy paxil 40 mg otc symptoms ringworm,73] (Figure 23 buy discount paxil 30mg on line medicine 5e. The other component was activated by a visceral afferent pathway in the pelvic nerve to facilitate voiding at the spinal micturition center [72]. Studies [71] in the anesthetized rat have further confirmed the seminal findings of Barrington [74]. At the initiation of micturition, intense vesical afferent activity activates the brainstem micturition center, which inhibits the spinal guarding reflexes (sympathetic and pudendal outflow to the urethra). Measurements of reflex bladder contractions under isovolumetric conditions during continuous urethral perfusion (0. Desensitization of the urethral afferent with intraurethral capsaicin also dramatically altered the micturition reflex. The existence of this pudendal nerve–mediated reflex has been confirmed as low-frequency electrical stimulation of afferent axons in the human pudendal nerve and the deep perineal nerve, a caudal branch of the pudendal nerve in cats that can initiate reflex bladder contractions and voiding [75,76]. The existence of urethra to bladder reflex may explain why stress incontinence and urge incontinence are comorbidities in women. Women with mixed incontinence may have detrusor overactivity activated by leakage of urine into the urethra due to stress incontinence, which support the theory of stress incontinence inducing urge incontinence [77]. Interestingly, surgical cure of the stress incontinence of women with mixed incontinence has resolved the urge incontinence in up to half of the patients. Inputs from supraspinal, spinal, and peripheral nervous system are necessary to maintain “switch-like” patterns of filling and voiding activity (Figures 23. The principal reflex components of these switching circuits are listed in Figures 23. Parasympathetic preganglionic axons that originate in the sacral spinal cord pass in the pelvic nerve to ganglion cells in the pelvic plexus and to distal ganglia in the organs. The pudendal and pelvic nerves also receive postganglionic axons from the caudal sympathetic chain ganglia. It is also possible that individual reflexes might be linked together in a serial manner to create complex feedback mechanisms. Thus, a bladder to sphincter to bladder reflex pathway could in theory contribute to the suppression of bladder activity during urine storage. Alterations in these primitive reflex mechanisms may contribute to neurogenic bladder dysfunction. Afferent Pathways 343 An intact afferent system is crucial to physiological voiding and bladder filling. During the filling phase, mechanoreceptors in bladder wall initiate visceral afferent (Aδ-fibers) activity that is carried by afferent axons in the pelvic nerve that synapse on spinal interneurons in lumbosacral spinal cord [62,80,81]. The pelvic nerve afferents consist of myelinated (A) and unmyelinated (C) axons to monitor the volume of the bladder and the amplitude of the bladder contraction (Figure 23. Visceral afferent fibers of the pelvic [82] and pudendal [83] nerves enter the cord and travel rostrocaudally within Lissauer’s tract. During neuropathic conditions and possibly inflammatory conditions, there is recruitment of C fibers from a new functional afferent pathway that can cause urge incontinence and possibly bladder pain. The suppression of detrusor overactivity in patients by sacral root stimulation may reflect in part the activation of the afferent limb of these visceral-bladder and somatic-bladder inhibitory reflexes [85–89]. Spinal and Supraspinal Pathways Involved in the Micturition Reflex In the spinal cord, afferent pathways terminate on second-order interneurons that relay information to the brain or to other regions of the spinal cord including the preganglionic sympathetic fibers from the intermediolateral cell column of the lower thoracic (T10) to upper lumbar (L2) spinal cord that form the thoracic and lumbar splanchnic nerves. Because polysynaptic pathways but not monosynaptic pathways mediate bladder, urethral, and sphincter reflex, interneuronal mechanisms play an essential role in the regulation of lower urinary tract function. Electrophysiological [90,91] and neuroanatomic [79,92–94] techniques have identified lower urinary tract interneurons in the same regions of the cord that receive afferent input from the bladder. Pharmacological experiments revealed that glutamic acid is the excitatory transmitter in these pathways [84]. Stimulation of afferent fibers from various regions (anus, colon–rectum, vagina, uterine cervix, penis, perineum, pudendal nerve) can inhibit the firing of sacral interneurons evoked by bladder distention [90]. This inhibition may be a result of presynaptic inhibition at primary afferent terminals or be due to direct postsynaptic inhibition of the second-order neurons. Direct postsynaptic inhibition of bladder preganglionic neurons can also be elicited by stimulation of somatic afferent axons in the pudendal nerve or visceral afferents from the distal bowel [95,96]. Peripheral Nervous System The lower urinary tract is innervated by three sets of the peripheral nerves involving the parasympathetic, sympathetic, and somatic nervous systems (Figure 23. Pelvic parasympathetic nerves arise at the sacral level of the spinal cord, excite the bladder, and relax the urethra. Lumbar sympathetic nerves inhibit the bladder body and excite the bladder base and urethra. Parasympathetic Pathways The preganglionic parasympathetic fibers arise from the ventral roots in the lateral part of the sacral intermediate gray matter in a region termed the sacral parasympathetic nucleus (S2–S4) and spinal cord to form the pelvic splanchnic nerves [63,82,84,98,99]. Upon coursing through and exiting the hypogastric and pelvic plexus, the fibers join the pelvic and pudendal nerves to synapse on terminal ganglia [1] and innervate the detrusor smooth muscle and urethra. The parasympathetic postganglionic neurons in humans are located not only in the detrusor wall layer but also in the pelvic plexus. This dual location can allow the possibility of afferent and efferent neuron interconnection at the level of the intramural ganglia in cauda equina or pelvic plexus injury [66,84]. Such injury leaves patients neurologically decentralized but may not be completely denervated. The activation of the sympathetic nerves induces relaxation of the bladder body and contraction of the bladder outlet and urethra, which contribute to filling phase in the bladder. Urine storage is facilitated by bladder wall relaxation and accommodation promoted by action of noradrenaline at β-adrenergic receptors in bladder [101] and activation of α- adrenergic receptors on the internal urethral sphincter resulting in contraction of the urethral outlet. Sphincter motoneurons also exhibit transversely oriented dendritic bundles that project laterally in the lateral funiculus, dorsally into the intermediate gray matter, and dorsomedially toward the central canal. Spinal reflex pathways not only enhance sympathetic outflow but also α-motoneuron discharge from Onuf’s nucleus. The inhibition can be induced by activation of afferent input from visceral organs including the penis, vagina, rectum, perineum, urethral sphincter, and anal sphincter [63,102,103]. Electrical stimulation of somatic afferent pathways projecting in the pudendal nerve to the caudal lumbosacral spinal cord can also inhibit voiding function. Electrophysiological studies in cats showed that the inhibition was mediated by suppression of interneuronal pathways in the sacral spinal cord and also by direct inhibitory input to the parasympathetic preganglionic neurons [104]. A similar inhibitory mechanism has been identified in monkeys by direct stimulation of the anal sphincter muscle [105]. Pontine Micturition Center and Brainstem Modulatory Mechanisms Various studies indicate that the micturition reflex is normally mediated by a spinobulbospinal reflex pathway passing through relay centers in the brain [61,62,66,78]. The occurrence of voiding dysfunction following lower thoracic spinal cord injury highlights the crucial role of supraspinal circuitry in voiding relative to the filling phase. The experiments involving brain-lesioning techniques in animals have confirmed that neurons in the brainstem at the level of the inferior colliculus have an essential role in the control of the parasympathetic component of micturition [61,62,66,108]. Removal of brain areas above the colliculus by intercollicular decerebration usually facilitates micturition by elimination of inhibitory inputs from more rostral centers. Although the circuitry in humans is uncertain, brain imaging studies have revealed increases in blood flow in this region of the pons during micturition [114].
Diseases
As long as the levator ani muscles function properly purchase paxil american express xerostomia medications that cause, the pelvic floor is closed and the ligaments and fascia are under no tension; the fasciae simply act to stabilize the organs in their position above the levator ani muscles cheap 40mg paxil overnight delivery treatment writing. When the pelvic floor muscles relax or are damaged buy paxil 20mg free shipping medications hydroxyzine, the pelvic floor opens and the vagina lies between the high abdominal pressure and low atmospheric pressure; in this situation, it must be held in place by the ligaments. Although the ligaments can sustain these loads for short periods of time, if the pelvic floor muscles do not close the pelvic floor, then the connective tissue must carry this load for long periods and will eventually fail to hold the vagina in place. Angles above the horizontal line have a “±” sign and those below the horizontal line a “−” sign. Thin lines indicate the portion of each force related to a closing and lifting function. The ship is analogous to the uterus, the ropes to the ligaments, and the water to the supportive layer formed by the pelvic floor muscles. The ropes (ligaments) function to hold the ship (uterus) in the center of its berth as it rests on the water (pelvic floor muscles). If, however, the water level were to fall so far that the ropes would be required to hold the ship without the support of the water, the ropes would break. The analogous situation in the pelvic floor involves the pelvic floor muscles supporting the uterus and vagina that are stabilized in position by the ligaments and fasciae: once the pelvic floor musculature becomes damaged and no longer holds the organs in place, the connective tissue fails because of significant overload. This lies at the level of the hymenal ring and attaches the urethra, vagina, and perineal body to the ischiopubic rami. Just above the perineal membrane are the compressor urethrae and urethrovaginal sphincter muscles, previously discussed as part of the striated urogenital sphincter muscle. Recent dissections show the intimate relationship between the perineal membrane and the levator ani muscle (Figure 21. The term “perineal membrane” replaces the old term “urogenital diaphragm,” reflecting more accurate recent anatomic information [56]. Previous concepts of the urogenital diaphragm show two fascial layers, with a transversely orientated muscle between them (the deep transverse perineal muscle). Observations based on serial histology and gross dissection, however, reveal a single connective tissue membrane, with these muscles and the levator ani muscles lying immediately above. Position and Mobility of the Urethra When the importance of urethral position to determining urinary continence was recognized, anatomic observations revealed an attachment of the tissues around the urethra to the pubic bones. These connections were referred to as the pubourethral ligaments [60] and were found to be continuous with the connective tissue of the perineal membrane [61]. Further studies [59,62,63] have expanded these observations and revealed several separate structural elements contained within these—tissues that have functional importance to urinary—continences [64]. A window in the perineal membrane has been cut to reveal the attachment of the levator ani muscle and its fusion with the vestibular bulb. Extension to the arcus tendineus fasciae pelvis is also shown, which is seen inside the pubic bone attaching to its inner surface, clitoris. Fluoroscopic and topographic observations [22,23] suggest that urethral position is determined both by attachments to the bone and by those to the levator ani muscles. The role of the connection between the ureteral supports and those to the levator ani is probably more important than previously thought for the following reasons: The resting position of the proximal urethra is high within the pelvis, some 3 cm above the inferior aspect of the pubic bones [65] (Figure 21. In addition, the upper two-thirds of the urethra is mobile [22,23,66] and under voluntary control. Although previously it was thought that support was the predominant factor, it is now clear that urethral function (maximal urethral closure pressure) is the primary determinant of whether or not a woman has stress incontinence [4]. Urethral support is probably more influential in younger women with stress urinary incontinence because they have relatively good urethral function. As urethral function declines with age, the role that maximum urethral closure pressure plays begins to predominate. In women with de novo stress incontinence after first birth, injury to the levator ani muscle is seen twice as often as individuals who deliver and are continent [67]. The anterior vaginal wall and urethra arise from the urogenital sinus and are intimately connected. The support of the urethra does not depend on attachments of the urethra itself to adjacent structures, but on the connection of the vagina and periurethral tissues to the muscles and fasciae of the pelvic wall. Surgeons are most familiar with seeing this anatomy through the space of Retzius, and this view is also helpful in understanding urethral support (Figure 21. The layer of tissue that provides urethral support has two lateral attachments: a fascial attachment and a muscular attachment (Figure 21. The muscular attachment connects these same periurethral tissues to the medial border of the levator ani muscle. These attachments allow the normal resting tone of the levator ani to maintain the position of the vesical neck, supported by the fascial attachments (Figure 21. When the muscle relaxes at the onset of micturition, it allows the vesical neck to rotate downward to the limit of the elasticity of the fascial attachments; at the end of micturition, contraction allows it to resume its normal position. Pubovesical muscle can be seen going from vesical neck to arcus tendineus fasciae pelvis and running over the paraurethral vascular plexus. Note that windows have been cut in the levator ani muscles, vagina, and endopelvic fascia so that the urethra and anterior vaginal walls can be seen. Also within this region are the pubovesical muscles, which are extensions of the detrusor muscle [1,68,69]. They lie within the connective tissue; when both muscular and fibrous elements are considered together, they are termed the “pubovesical ligaments,” in much the same way that the smooth muscle of the ligamentum teres is referred to as the round ligament (see Figures 21. Although the terms “pubovesical ligament” and “pubourethral ligament” have sometimes been considered to be synonymous, the pubovesical ligaments are different structures from the urethral supportive tissues. Fibers of the detrusor muscle are able to undergo great elongation, and these weak tissues are, therefore, not suited to maintain urethral position under stress. In addition, they run in front of the vesical neck rather than underneath it, where one would expect supportive tissues to be found. It is not surprising, therefore, that these detrusor fibers do not differ, in stress-incontinent patients, from those in patients without this condition [70]. The tissues that support the urethra are separated from the pubovesical ligaments by a prominent vascular plexus and are easily parted from them. Rather than supporting the urethra, the pubovesical muscles may be responsible for assisting in vesical neck opening at the onset of micturition by contracting to pull the anterior vesical neck forward, as some have suggested [71]. This mechanism influences incontinence by determining how the urethra is supported, not by how high or low the urethra is in the pelvis. In examining anatomic specimens, simulated increases in abdominal pressure reveal that the urethra lies in a position where it can be compressed against the supporting hammock by rises in abdominal pressure (Figure 21. In this model, it is the stability of this supporting layer under the urethra rather than the height of the urethra that determines stress continence. In an individual with a firm supportive layer, the urethra would be compressed between abdominal pressure and pelvic fascia (Figure 21.